Unlocking the Science Behind Car Battery Conversions: How Kinetic Energy Turns into Electrical Energy
Have you ever found yourself stuck with a dead car battery and no nearby store selling a replacement battery? It can be a frustrating and stressful situation, especially if you’re running late for an important appointment. But what if there was a way to convert your existing car battery into a more long-lasting, efficient power source? This is where car battery conversion comes in. By making a few modifications to your existing battery, you can increase its capacity and extend its lifespan, saving you both time and money.
In this blog post, we’ll be discussing the benefits of car battery conversion and how it can improve your driving experience. So buckle up and let’s dive in!
The Science Behind Electrical Energy
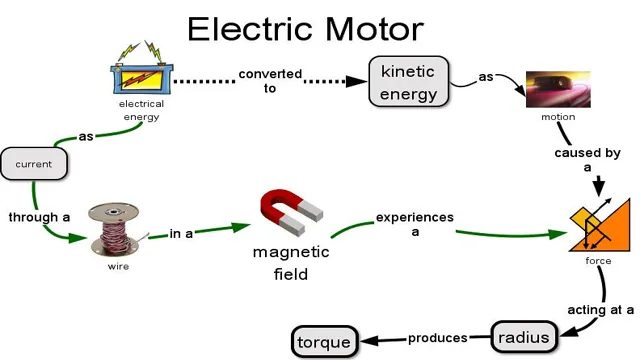
When it comes to car batteries, many people wonder how they are able to convert energy into electrical energy. The process involves a chemical reaction that takes place within the battery itself. Specifically, the battery contains two electrodes – a positively charged anode and a negatively charged cathode – which are separated by an electrolyte.
When the battery is connected to an electrical device, a chemical reaction takes place that causes electrons to transfer from the anode to the cathode, creating an electrical current. This current can then be used to power a variety of devices, from headlights to radios to air conditioning systems. Essentially, the car battery is able to convert chemical energy into electrical energy, enabling your car to function properly.
Chemical Energy to Electrical Energy
Chemical energy is a fundamental concept in the production of electrical energy. In simplistic terms, chemical energy refers to the energy stored in chemical bonds that hold molecules together. This energy can be released through a chemical reaction, which can then be harnessed to generate electrical energy.
This is the principle behind batteries, which utilize a chemical reaction to produce electricity. Basically, electrical energy is made possible by the flow of electrons. In a battery, the chemical reaction creates a flow of electrons, which can be used to power electrical devices.
More complex electrical energy generation systems, like power plants, utilize chemical reactions to generate heat, which is then transformed into electrical energy through the use of generators. So, in short, chemical energy is a vital component in the generation of electrical energy.
Electrical Energy Powers Your Car
Electrical energy is what powers your car’s engine, and understanding the science behind it can help explain why your vehicle can zoom down the road. At its core, electrical energy is simply the movement of electrons through a conductor. In your car, the alternator is responsible for generating electrical energy by converting mechanical energy from the engine into electrical energy, which then charges the battery.
This electrical energy is then sent to the spark plugs, which ignite the fuel and air mixture to power the engine. It’s a complex system of energy conversion, but without it, your car wouldn’t be able to move. Just like a river flowing downstream, electrical energy follows the path of least resistance, making its way through the various electrical components of your car.
By harnessing this electrical energy, your car can move with great speed and efficiency, transporting you to your destination in no time.
How Car Batteries Actually Work
When it comes to your car’s battery, have you ever wondered how it actually works? Well, car batteries are rechargeable, and they convert chemical energy into electrical energy in order to power your vehicle’s electrical components. Specifically, the battery utilizes a simple electrolysis process: lead plates and lead oxide plates are immersed in a solution of water and sulfuric acid. This creates a chemical reaction that generates electrons, which are the basic units of electricity.
These electrons travel through the battery’s acid, creating a flow of electrical current. This electrical current is then used to power your vehicle’s starter motor, lights, sound system, and other electrical components. Over time, as you use your car battery, the chemical reaction that generates electricity diminishes, eventually leading to the need for a replacement battery.
Make sure to keep your battery charged and in good condition to ensure proper functioning of your vehicle’s electrical components.
Electrons and Electrolytes
Car batteries are often taken for granted, but they play a crucial role in the operation of our vehicles. At the heart of a car battery is a series of chemical reactions that generate electrical energy using electrons. The battery contains two electrodes: a positive cathode and a negative anode, which sit in an electrolyte solution.
When the car is turned on, the alternator generates electricity that flows into the battery and causes a chemical reaction that releases electrons from the anode. These electrons flow through a circuit and reach the cathode, where they are absorbed. This process continues back and forth until the battery becomes depleted of electrons and needs to be recharged.
The electrolyte solution helps facilitate the chemical reactions by allowing the flow of ions between the electrodes. Essentially, car batteries are like a miniature power plant that converts chemical energy into electrical energy, providing the power needed to start our cars and operate their electrical systems.
How the Battery Generates a Charge
Car battery When you turn the key in your car’s ignition, it sends an electrical signal to your car’s battery. This signal initiates the battery’s chemical reaction that generates an electric charge. Essentially, a car battery is made up of two electrodes: a negative electrode (also known as the anode) and a positive electrode (also known as the cathode).
These electrodes generate an electric current by transferring electrons between them through an electrolyte solution. This electrolyte is a mixture of sulfuric acid and water, which allows for the transfer of ions between the two electrodes. When the battery is fully charged, the negative electrode is made up of lead, while the positive electrode is made up of lead oxide.
When the battery is in use, the lead oxide electrode gives up electrons while the lead electrode receives them, generating an electric current. This current is then sent through your car’s electrical system to start the engine, power your lights, and operate your accessories. It’s essential to take care of your car battery by maintaining its charge level and checking its water levels regularly to ensure it works efficiently.
Battery Charging and Discharging
Car batteries are an essential component of our vehicles, as they provide the power needed to start the engine and power other electrical systems. These batteries operate using a chemical reaction between lead and sulfuric acid, which produces electrical energy. When the battery is charged, electrical current flows into the battery, causing the lead and sulfuric acid to react and generate energy.
On the other hand, when the battery is discharged, the chemical reaction slows down, and the energy stored in the battery is depleted. In order to keep the battery charged, your car’s alternator continuously charges the battery while the engine is running. The alternator works by converting the engine’s mechanical energy into electrical energy, which is then used to charge the battery.
However, if the alternator fails, the battery will eventually become fully discharged and cannot power the car’s electrical system. This is why it’s crucial to keep your vehicle’s battery in good condition by ensuring it’s regularly charged and tested. To summarize, car batteries work by utilizing a chemical reaction between lead and sulfuric acid to generate electrical energy.
They need to be charged regularly to maintain their ability to provide power to the vehicle’s electrical system. By understanding how car batteries work and keeping them maintained, you can ensure that your vehicle is ready to start when you need it most.
The Importance of a High-Quality Car Battery
A car battery is an essential component of every vehicle. It plays a crucial role in providing electrical energy to start the engine, power the lights, and other electronic devices within the vehicle. But, what does a car battery convert into electrical energy? Well, it is the chemical energy stored inside the battery that gets converted into electrical energy to power the vehicle.
The battery employs a chemical reaction between lead plates and acid to generate electricity. This process produces electrons that flow in a circuit to power all the electrical components of a vehicle. Therefore, having a high-quality car battery is vital for ensuring that the car runs smoothly and efficiently.
A subpar battery may not provide enough power to start the vehicle, especially during cold weather conditions, leading to inconvenience and costly repairs. Regular maintenance and timely replacement of a car battery can also prevent battery-related breakdowns, improve fuel efficiency, and prolong the life of the vehicle’s electrical system.
Reliability and Efficiency
When it comes to a car’s reliability and efficiency, the battery is a crucial component that can’t be overlooked. A high-quality car battery is essential for ensuring that your vehicle starts up every time you turn the key, even in extreme weather conditions. It’s not just about getting from point A to point B, but also about avoiding the frustration and inconvenience of a dead battery.
A good battery also helps keep your car’s electrical system running smoothly, allowing all the other components to work together seamlessly. Think of a battery as the heart of your car, pumping life into everything else. Neglecting your battery or settling for a cheap, low-quality option can end up costing you more in the long run.
Invest in a reliable battery and enjoy the peace of mind that comes with knowing your car is always ready to go.
Safety and Environmental Concerns
As a responsible car owner, it’s crucial to prioritize safety and environmental concerns when it comes to maintaining your vehicle. Choosing a high-quality car battery from a reputable brand is one way you can help ensure your vehicle operates safely while also minimizing its impact on the environment. An inferior car battery can be prone to issues like leaks and explosions, which can be a serious safety hazard for you and others on the road.
Additionally, cheap batteries may not be as energy-efficient, which can result in more frequent replacements and unnecessary waste. Opting for a well-made car battery can not only improve your driving experience but also reduce your carbon footprint. So, the next time you’re in the market for a new battery, invest in a high-quality option that will help keep you safe and protect the environment.
Conclusion: Keeping Your Car Running Smoothly and Safely
So, as it turns out, a car battery is like a magician’s trick box, converting the potential energy stored in its chemistry into the electrical energy that powers your car. It’s like a superhero that never quits, providing constant energy for your car’s many electrical systems. And just like a trusty sidekick, the battery also works in tandem with the alternator to keep the electricity flowing, ensuring that your car never runs out of juice.
So the next time you turn that key and your car comes to life, take a moment to thank your battery for its electrifying performance.”
FAQs
What is a car battery?
A car battery is a rechargeable battery that provides electrical energy to a motor vehicle.
How does a car battery work?
A car battery converts chemical energy stored in its cells into electrical energy when needed to power the vehicle’s starter motor.
What type of energy does a car battery convert into electrical energy?
A car battery converts chemical energy into electrical energy.
How long does a car battery last?
The lifespan of a car battery can depend on various factors such as usage, climate, and maintenance. However, on average, a car battery can last between 2 to 5 years.
Can a car battery be recharged?
Yes, a car battery can be recharged using an external charger or by driving the vehicle to allow the alternator to recharge the battery as it runs.