The Revolutionary Chemistry Behind Electric Car Batteries: A Breakthrough in Sustainable Transportation
Electric cars have gained immense popularity in recent years. Their eco-friendliness, low emission levels, and energy efficiency make them a viable option for the future of transportation. But have you ever wondered, what makes these cars run without gasoline? The answer lies in their batteries, which are made up of multiple chemical components.
The chemical components of electric car batteries play a crucial role in determining the range, lifespan, and overall performance of these vehicles. In this blog, we’ll take a closer look at the various chemical components that make up an electric car battery, how they work together, and their impact on the environment. So, let’s dive in and uncover the science behind these innovative and sustainable machines.
Lithium-Ion Batteries
When it comes to electric car batteries, one of the most important chemicals used is lithium-ion. These batteries have revolutionized the way we power our vehicles, providing a cleaner and more sustainable solution compared to traditional gasoline engines. Lithium-ion batteries are able to store more energy and deliver it more efficiently than their predecessors, making them an ideal choice for electric cars.
They work by utilizing lithium ions to move between a cathode and an anode, generating an electrical charge that powers the car. This simple yet effective process has made electric cars more accessible to consumers, and with advancements in technology, these batteries are becoming even more efficient and durable. As demand for electric cars grows, it’s likely that lithium-ion batteries will continue to play a central role in powering the transportation industry.
Composition and Functionality
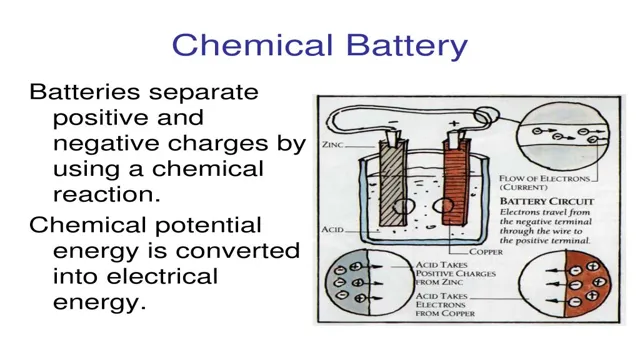
Lithium-ion batteries are a type of rechargeable battery that use lithium ions as the key component. These batteries consist of two electrochemical cells, typically made up of a positive cathode and a negative anode, separated by a porous material called a separator. The cathode is usually made of lithium metal oxide, while the anode is made of graphite.
During discharge, lithium ions move from the cathode to the anode through the separator, generating an electric current that powers devices such as smartphones and laptops. When the battery is recharged, the process is reversed and lithium ions move back to the cathode. Lithium-ion batteries are known for their high energy density, long lifespan, and ability to retain their charge for long periods of time, making them a popular choice in the consumer electronics industry.
Advantages over Other Battery Types
When compared to other battery types, lithium-ion batteries have several advantages that make them a popular choice for many applications. One of the biggest advantages is their high energy density, which means they can store more energy in a smaller size than other battery types. This makes them ideal for use in portable devices like smartphones and laptops, where space is limited.
Lithium-ion batteries also have a long lifespan and can be recharged hundreds of times before they start to degrade. Additionally, they are relatively lightweight and have a low self-discharge rate, which means they can hold a charge for a long time without losing their energy. Overall, these benefits make lithium-ion batteries a reliable and efficient choice for a wide range of uses.
Nickel-Metal Hydride (NiMH) Batteries
When it comes to the chemical makeup of electric car batteries, Nickel-Metal Hydride (NiMH) batteries are a popular choice. These batteries use hydrogen-absorbing alloys to store energy, making them a more environmentally-friendly alternative to their lead-acid counterparts. They have a higher energy density, allowing them to store more energy in a smaller space, and they can be recharged hundreds of times before needing to be replaced.
However, they do have some downsides, such as decreased performance in extreme temperatures and a higher self-discharge rate. Despite these limitations, NiMH batteries continue to be used in electric and hybrid vehicles as they strike a balance between efficiency, affordability, and sustainability. Overall, the use of this chemical in car batteries represents a step forward in the development of cleaner and more efficient modes of transportation.
Composition and Functionality
Nickel-Metal Hydride (NiMH) batteries are rechargeable batteries that use a nickel-hydrogen positive electrode and a hydrogen-absorbing negative electrode. The electrolyte used in these batteries is potassium hydroxide, which facilitates the movement of ions between the electrodes. NiMH batteries have a higher energy density and can store more power than their predecessor, nickel-cadmium batteries.
They also have a longer cycle life and are less prone to memory effect. This makes them a popular choice for consumer electronics, such as digital cameras and toys, as well as hybrid and electric vehicles. NiMH batteries are environmentally friendly as they do not contain harmful metals like cadmium and mercury.
However, they are not as efficient in terms of power output compared to newer batteries like lithium-ion. Overall, NiMH batteries are a reliable and cost-effective option for many everyday applications.
Advantages and Disadvantages Compared to Lithium-Ion Batteries
Nickel-Metal Hydride (NiMH) batteries are a popular alternative to lithium-ion batteries, especially for low-power applications. One of the major advantages of NiMH batteries is that they are cheaper than lithium-ion batteries. Additionally, NiMH batteries have a higher energy density than other types of rechargeable batteries, allowing them to store more energy in a smaller space.
This makes them ideal for use in portable electronic devices such as cameras, toys, and handheld gaming consoles. However, NiMH batteries also have some disadvantages. They have a lower voltage than lithium-ion batteries, which can limit the power output of devices that use them.
They are also prone to self-discharge, which means they lose energy over time even when not in use. Nonetheless, NiMH batteries remain a popular choice for many applications where cost and energy density are more important than power output and longevity.
Lead-Acid Batteries
Did you know that lead-acid batteries are commonly used in electric cars? This chemical combination has been used for over a hundred years, making it a reliable source of power. Lead and sulfate are used to create an electrochemical reaction that produces energy, which is stored in the battery. However, lead-acid batteries have some downsides, such as being heavy and having a limited lifespan.
With the advancements in technology, manufacturers are seeking alternative options to improve the efficiency of electric car batteries. Nonetheless, lead-acid batteries remain a popular choice because they are cost-effective and readily available. So next time you drive an electric car, keep in mind that lead-acid batteries are powering it.
Composition and Functionality
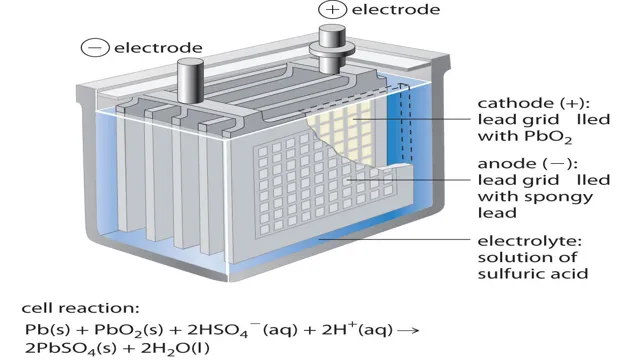
Lead-acid batteries are some of the oldest and most reliable batteries available. They consist of lead plates and an electrolyte solution of sulfuric acid and water. The lead plates are coated with lead oxide, which reacts with the sulfuric acid to produce lead sulfate.
The process of discharging reverses this reaction, creating electricity. Unlike lithium-ion batteries, lead-acid batteries are highly resistant to overcharging and overheating, making them ideal for use in automobiles, boats, and backup power systems. While these batteries are heavier and bulkier than other options, their low cost and durability make them a popular choice for many applications.
When it comes to lead-acid batteries, a key consideration is the ability to properly maintain and recharge them in order to ensure their longevity and optimal performance.
Comparison with Lithium-Ion Batteries
When it comes to comparing lead-acid batteries with lithium-ion batteries, there are a few noticeable differences to consider. First and foremost, lead-acid batteries are typically much cheaper than their lithium-ion counterparts. However, lead-acid batteries are heavier, bulkier, and have a shorter lifespan.
On the other hand, lithium-ion batteries are more expensive upfront, but they’re lighter, smaller, and have a longer lifespan. Additionally, lithium-ion batteries tend to have a higher energy density than lead-acid batteries, meaning they can store more energy in a smaller space. It’s important to note that lead-acid batteries are still commonly used in vehicles due to their affordability and ability to deliver high currents, whereas lithium-ion batteries are more commonly used in consumer electronics due to their improved energy density and longer lifespan.
Ultimately, the decision between lead-acid and lithium-ion batteries comes down to the intended use and specific needs of the user.
Future Developments in Electric Car Battery Technology
One of the most pressing issues in the world of electric cars is the development of new, more efficient battery technologies. Currently, the most popular type of electric car battery is the lithium-ion variety, which uses a chemical reaction to store and release energy. However, researchers are constantly looking for ways to improve this technology, with many focusing on developing new types of chemical reactions that can generate more power and last longer.
Some believe that solid-state batteries, which do not use liquid electrolytes, have great potential in this regard, while others are looking at lithium-oxygen batteries that can potentially offer even higher energy densities. While these technologies are still in development, they could be game-changers for the world of electric cars, making them even more practical and energy-efficient than they already are.
New Materials Being Explored and their Potential Benefits
Electric Car Battery Technology Electric cars are becoming more popular as environmental concerns grow, and researchers are constantly seeking to improve battery technology. One promising development is the use of new materials, such as solid-state batteries, which have the potential to be safer, more efficient, and longer-lasting than traditional lithium-ion batteries currently used in most electric cars. Solid-state batteries offer higher energy density and faster charging times, which could eliminate one of the primary challenges of electric cars: range anxiety.
In addition, scientists are also exploring the use of alternative materials, such as silicon or sulfur, which could lead to even more efficient batteries that can store more energy and reduce their reliance on rare and expensive metals like cobalt. While these developments are still in the experimental stage, they suggest a promising future for battery technology and electric cars.
Challenges in Developing and Commercializing Advanced Battery Technologies
The development of advanced battery technologies is an exciting frontier that holds significant potential for the future of sustainable transportation. The electric car battery market is rapidly evolving, and innovation is essential to stay ahead of the curve. Battery manufacturers and automakers face the challenges of improving battery performance, reliability, safety, and durability while keeping costs down and reducing environmental impacts.
Some of the latest developments in battery technology include solid-state batteries, which offer higher energy density and improved safety compared to traditional lithium-ion batteries. Additionally, researchers are exploring new materials and manufacturing processes that could lead to longer-lasting and more efficient batteries. The race to commercialize advanced battery technologies is intensifying, and the winners will be those that can meet the demands of consumers, regulators, and investors while driving down the costs of electric cars and boosting their performance.
Conclusion
In conclusion, it’s clear that chemistry plays a crucial role in the functioning of an electric car battery. From the electrolytes to the anodes and cathodes, every aspect of the battery’s composition is carefully designed to optimize its performance and energy efficiency. So next time you take a ride in an electric car, remember that beneath the sleek exterior lies a complex chemical powerhouse that helps reduce carbon emissions and make our world a cleaner and more sustainable place!”
FAQs
How do chemicals play a role in electric car batteries?
Chemicals are used in electric car batteries as a way to store and produce energy. They are responsible for the conversion of electrical energy to chemical energy and vice versa.
What chemicals are most commonly used in electric car batteries?
The most common chemicals used in electric car batteries are lithium-ion, nickel-metal hydride, and lead-acid.
How do lithium-ion batteries compare to other types of batteries in terms of efficiency?
Lithium-ion batteries are more efficient than other types of batteries because they have a higher energy density, meaning they can store more energy in a smaller and lighter battery.
Are there any environmental concerns associated with the chemicals used in electric car batteries?
Yes, there are concerns about the mining and disposal of the chemicals used in electric car batteries. However, efforts are being made to find more sustainable and eco-friendly alternatives.