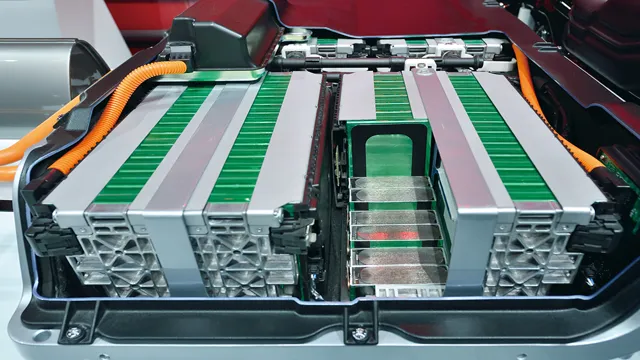
Revolutionizing the Future: The Groundbreaking Electric Car Battery Made Up Of [Insert Material]
Electric cars have been making headlines in recent years due to their eco-friendly benefits and lower emissions. One of the most vital components of an electric car is the battery, which is responsible for powering the vehicle. But what exactly are these batteries made of? In this blog post, we’ll delve deeper into the components of electric car batteries and explore their specific functions.
From metals and electrolytes to electrodes and separators, we’ll uncover the science behind these powerful batteries that are paving the way for a sustainable automotive industry. So, join us as we take a closer look at the composition of electric car batteries and find out what makes them tick.
Lithium-Ion Cells
Electric car batteries are typically made up of lithium-ion cells. These cells are made up of a cathode, an anode, and an electrolyte. The cathode is typically made up of a metal oxide, such as lithium cobalt oxide or lithium iron phosphate, while the anode is usually made of graphite.
The electrolyte, which is the medium that allows the movement of ions from one electrode to the other, is typically a lithium salt in an organic solvent. One of the main reasons why lithium-ion cells are used in electric car batteries is because they have a high energy density, which means that they can store a large amount of energy in a relatively small space. This is important for electric cars because they need to be able to travel long distances on a single charge.
Lithium-ion cells also have a long cycle life, which means that they can be charged and discharged many times before they start to deteriorate. However, there are also some downsides to using lithium-ion cells in electric car batteries. One of the main concerns is safety, as they have been known to catch fire or explode if they are damaged or overheated.
Another issue is that they can be expensive to manufacture, which can make electric cars more expensive than their gasoline-powered counterparts. Despite these challenges, lithium-ion cells remain the most popular choice for electric car batteries, and researchers continue to look for ways to improve their safety, reliability, and cost-effectiveness. As a result, we can expect to see even more electric cars on the road in the coming years, helping to reduce our reliance on fossil fuels and improve air quality in our cities.
Composition of Lithium-Ion Cells
Lithium-ion cells are the building blocks that make up the batteries that power many of our electronic devices. These cells are made up of several components including electrodes, electrolyte solutions, and separators. The anode, or positive electrode, is typically made of graphite while the cathode, or negative electrode, is made up of lithium cobalt oxide, lithium iron phosphate, or similar materials.
The electrolyte solution is generally a combination of lithium salts and organic solvents like ethylene carbonate and dimethyl carbonate. It is responsible for carrying the lithium ions between the electrodes during charging and discharging cycles. The separator is a thin polypropylene or similar material that serves as a barrier between the electrodes to prevent electrical short circuits.
The combination of these components determines the performance and safety of the lithium-ion cell, and manufacturers carefully engineer and test them to optimize their composition. Overall, lithium-ion cells are complex and intricate entities that enable modern technology to operate efficiently and reliably.

Advantages of Lithium-Ion Batteries
Lithium-ion cells are highly advantageous compared to traditional batteries. They are smaller, lighter, and more efficient, making them an ideal choice for various electronic devices. Lithium-ion cells recharge quickly and provide a longer lifespan, enabling them to power devices for extended periods.
They are also less environmentally harmful than traditional batteries, reducing the carbon footprint of many industrial processes. What’s more, lithium-ion cells offer better performance, making them an excellent choice for electric vehicles and renewable energy storage. Although they may be slightly more expensive than traditional batteries, lithium-ion cells provide better value in terms of performance, lifespan, and eco-friendliness.
In conclusion, lithium-ion cells offer numerous advantages that make them a popular choice for various applications, whether for personal or industrial purposes.
Cathode and Anode Materials
An electric car battery is made up of cathode and anode materials. These materials play a crucial role in the battery’s overall performance since they are responsible for storing and releasing electrical energy. The cathode, typically made of metal oxide, is the positive electrode where the movement of ions takes place.
In contrast, the anode, commonly made of graphite, is the negative electrode that attracts positively charged ions. When the battery is in use, the lithium ions move from the anode to the cathode, creating a flow of electrical current that powers the vehicle. Because the efficiency of an electric car battery is dependent on the materials used in the cathode and anode, researchers are continually exploring new options that can improve performance and energy storage.
Some promising developments include the use of alternative materials such as sulfur and silicon, which could lead to longer-lasting batteries with greater capacity. As electric vehicles become increasingly popular, the demand for more efficient and effective battery materials will only continue to grow.
Popular Cathode Materials Used in Electric Car Batteries
Cathode materials play a crucial role in the performance of electric car batteries. There are several popular cathode materials being used in modern electric vehicles, with each having their own unique properties and capabilities. One of the most commonly used cathode materials is lithium cobalt oxide, or LCO.
LCO has a high energy density, allowing for longer driving ranges, but it can be unstable and prone to overheating if not properly managed. Another popular cathode material is nickel cobalt aluminum oxide, or NCA, which is known for its high energy density and long lifespan. However, NCA can be expensive and difficult to manufacture.
A third popular cathode material is lithium nickel manganese cobalt oxide, or NMC, which offers a good balance between cost, energy density, and lifespan. NMC cathodes are widely used in electric vehicles and have proven to be a reliable and effective option. Overall, the choice of cathode material depends on a variety of factors, including cost, energy density, and safety considerations.
By carefully selecting the right cathode material, electric car manufacturers can create high-performance batteries with longer driving ranges and better overall efficiency.
Popular Anode Materials Used in Electric Car Batteries
Popular Anode Materials Used in Electric Car Batteries When it comes to electric car batteries, not all materials are created equal. The two main components of the battery are the cathode and the anode. While the cathode is responsible for storing the majority of the energy, the anode plays a crucial role in the battery’s overall performance and longevity.
Some popular anode materials used in electric car batteries include graphite, silicon, and lithium. Graphite is the most commonly used material because it is lightweight, inexpensive, and has a high electrical conductivity. Silicon is a promising alternative, with a much higher energy density than graphite, but it can be expensive and difficult to produce in large quantities.
Finally, lithium is another material that can be used as an anode but is typically only used in specialty batteries due to its high cost and potential safety concerns. Ultimately, the choice of anode material will depend on factors such as cost, energy density, and safety considerations. By selecting the right anode material, manufacturers can ensure that electric car batteries are both efficient and reliable.
Trade-Offs in Cathode and Anode Material Selection
Cathode and Anode Materials Selecting the right cathode and anode materials is crucial for the successful functioning of batteries. These materials play a critical role in determining a battery’s capacity, energy density, lifetime, and performance. However, there are several trade-offs to consider when selecting these materials, such as cost, safety, availability, and environmental impact.
For cathode materials, options range from cheaper and widely available materials like nickel, cobalt, and manganese to more expensive but more stable and long-lasting materials like lithium and iron. Similarly, for anode materials, carbon-based materials are the most widely used, but silicon, tin, and metal alloys are also showing promise. The choice of materials will depend on the specific application and desired battery performance.
It’s important to consider these trade-offs and conduct thorough research before making a final decision on cathode and anode materials in order to produce a battery that meets both technical and economic requirements.
Electrolyte and Separator Materials
An electric car battery is made up of several key components, including the electrolyte and separator materials. These materials play a crucial role in the overall performance and longevity of the battery. The electrolyte is the medium through which ions move between the cathode and anode, generating the electric current needed to power the vehicle.
It is typically composed of lithium salts dissolved in an organic solvent, and must be carefully formulated to ensure optimal conductive properties and stability over time. The separator, meanwhile, is a thin layer that physically separates the cathode and anode to prevent short circuits, while still allowing ions to pass through. Materials commonly used for separators include polymer films and ceramic membranes, which must be carefully engineered to meet the demanding requirements of electric vehicle applications.
By selecting high-quality electrolytes and separators, battery manufacturers can create electric car batteries with longer range, faster charging times, and better overall performance.
Purpose and Function of Electrolyte and Separators
Electrolyte and Separator Materials When it comes to batteries, electrolyte and separator materials play a critical role in their overall function and performance. The electrolyte serves as the conductive medium that allows ions to travel between the battery’s positive and negative electrodes. This process creates an electrical current that can be harnessed for various applications.
Separator materials, on the other hand, prevent the electrodes from making contact and short-circuiting the battery. They also allow ions to pass through while preventing the mixing of the electrolyte and the creation of unwanted reactions that can degrade a battery’s performance or cause it to fail altogether. There are a variety of electrolyte and separator materials available, each with its own unique properties and advantages.
For example, some electrolytes are liquid-based, while others are solid-state. Similarly, separators can be made from a range of materials such as polyethylene, polypropylene, and ceramics. Choosing the right electrolyte and separator materials depends on the specific application and desired performance characteristics.
In recent years, there has been a growing interest in developing more environmentally friendly and sustainable electrolyte and separator materials that can help reduce the environmental impact of battery production and disposal.
Common Electrolytes Used in Electric Car Batteries
When it comes to electric car batteries, electrolytes and separator materials play a crucial role. Electrolytes are necessary for the movement of ions between the electrodes, which then creates a flow of electrical energy. Commonly used electrolytes in electric car batteries include aqueous electrolytes, organic electrolytes, and solid-state electrolytes.
Aqueous electrolytes are water-based and are commonly used in lead-acid batteries. Organic electrolytes, on the other hand, are typically used in lithium-ion batteries and can be combined with various salts to enhance their performance. Solid-state electrolytes are non-liquid materials that can conduct ions and are known for their high safety and long lifespan.
Separator materials are also important components of the battery as they prevent direct contact between the anode and cathode, which helps to prevent short-circuits. Common separator materials include microporous polyolefin membranes, ceramic-coated separators, and separator papers. By selecting the appropriate electrolyte and separator materials, electric car batteries can provide high-performance and a longer lifespan.
Battery Management Systems
If you’ve ever wondered what an electric car battery is made up of, the answer is a combination of many smaller cells that work together to power the vehicle. However, managing these cells can be a complex process, which is why electric cars rely on battery management systems (BMS) to ensure optimal performance and safety. These sophisticated systems monitor the battery’s charge level, temperature, and other factors to prevent overcharging or overheating that could result in damage to the battery or even a fire.
They also balance the cells’ charge levels to ensure that the entire battery is used evenly, maximizing its lifespan and performance. With advances in BMS technology, electric cars are becoming more reliable and efficient, making them an increasingly popular choice for eco-conscious drivers.
Importance of Battery Management Systems in Electric Vehicles
As electric vehicles continue to gain popularity, the importance of battery management systems (BMS) cannot be overstated. A BMS is a vital component that oversees the health of the battery, including monitoring temperature, voltage, and current. It helps to prolong the battery lifespan by preventing overcharging and over-discharging, balancing the cells, and identifying any abnormalities.
A faulty BMS can lead to reduced battery life, performance issues, and even safety hazards. Effective battery management is crucial for maximizing the range of electric vehicles and reducing the cost of maintenance. Without a functional BMS, the battery could fail prematurely, leaving the vehicle stranded.
Therefore, a reliable BMS is essential for the successful integration of electric vehicles into our everyday lives. With the advancements in technology, battery management systems are becoming more sophisticated, allowing for greater efficiency and reliability. As a result, the future of electric vehicles looks promising, and BMS plays a significant role in making this a reality.
Conclusion
In conclusion, an electric car battery is like a mini power plant that’s made up of a complex network of cells, modules, and electronics that work together to power our eco-friendly vehicles. Imagine a jigsaw puzzle where each piece plays a vital role in creating the bigger picture. From the cathode and anode to the electrolyte and separator, the key to building the ultimate electric car battery is combining all of these components in the most efficient and effective way possible.
So, next time you’re cruising down the road in your fully electric car, remember that behind the quiet hum of your vehicle, there’s a sophisticated battery system working tirelessly to keep you moving forward. “
FAQs
What materials are used to make the electric car battery?
Electric car batteries are typically made up of lithium-ion, nickel-metal hydride, or lead-acid cells.
How long do electric car batteries typically last?
The lifespan of an electric car battery depends on various factors, including the specific battery technology used and the driving habits of the owner, but they generally last between 5-10 years.
Can electric car batteries be recycled?
Yes, electric car batteries can be recycled, and many manufacturers offer take-back programs to ensure proper disposal of used batteries.
How does the range of an electric car battery compare to a gas-powered vehicle?
The range of an electric car battery varies depending on the specific vehicle and battery technology, but on average, they have a lower range compared to gas-powered vehicles. However, advances in battery technology and charging infrastructure have made range anxiety less of a concern for electric car owners.