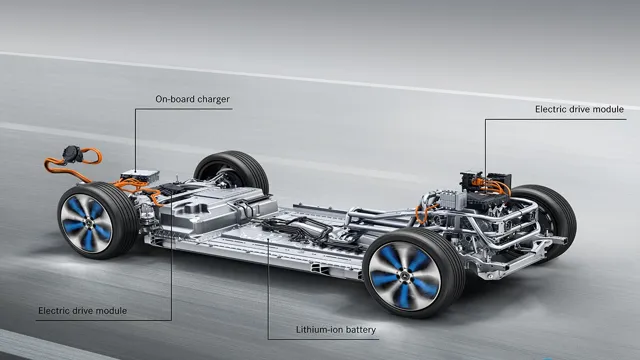
The electrifying chemistry behind the power-packed batteries in electric cars
If you’re one of the many drivers who’ve chosen to make the switch to an electric car, you’ve probably wondered about the science behind the battery that powers your vehicle. While it may seem like magic, the truth is that the chemistry of electric car batteries is actually quite fascinating. At its heart, an electric car battery is simply a sophisticated version of the batteries you use in your everyday life – like the ones in your remote control or your cellphone.
But while those batteries use an acid solution to generate electricity, the batteries in electric cars rely on advanced chemical reactions to generate power. These reactions involve a combination of lithium ion cells, complex cathodes, and highly conductive electrolytes. Together, these elements work to create the chemical reaction that generates the electrical current that powers your car.
And because electric cars rely solely on battery power, these batteries need to be not only highly reliable and efficient, but durable enough to last for many years. So how does it all work, exactly? In this blog, we’ll delve deep into the chemistry behind electric car batteries, exploring the different components that make up these powerful energy sources and examining the complex chemistry that makes them possible. Along the way, we’ll look into some of the latest advancements in battery technology and explore what they mean for the future of electric cars.
Ready to learn more about the inner workings of your electric car battery? Keep reading to find out everything you need to know about this fascinating technology.
What Makes Electric Car Batteries Different?
The chemistry behind electric car batteries is quite fascinating. Unlike traditional lead-acid batteries that rely on the transfer of ions between two electrodes, electric car batteries use lithium-ion technology. This means that lithium ions move from one electrode to the other through an electrolyte solution, generating an electric charge in the process.
However, not all lithium-ion batteries are created equal. The batteries used in electric cars have a higher energy density and longer cycle life than those used in consumer electronics. This is because they are made of more durable materials and have a more complex structure.
Electric car batteries need to be able to store a lot of energy and deliver it efficiently to power the vehicle. To achieve this, they are made up of multiple cells that are interconnected and controlled by a complex battery management system.
Lithium-ion Batteries vs. Lead-acid Batteries
When it comes to electric cars, the battery is a crucial component that directly impacts the performance and functionality of the vehicle. Two of the most common types of electric car batteries are lithium-ion and lead-acid batteries. Lithium-ion batteries are the more popular choice due to their higher energy density, longer lifespan, and lighter weight compared to lead-acid batteries.
Unlike lead-acid batteries, lithium-ion batteries can recharge faster and offer better acceleration for electric vehicles. Plus, they are more eco-friendly since they do not contain harmful materials such as lead and acid. Overall, lithium-ion batteries are a superior choice for electric cars since they provide better performance, reliability, and sustainability.
How Do Electric Car Batteries Work?
When you plug in an electric car, you’re charging up its lithium-ion battery, but how exactly does that battery work? The chemistry behind an electric car battery is fascinating. Unlike traditional fuel-powered vehicles, electric cars don’t have an engine. Instead, their batteries store electrical energy, which powers an electric motor that propels the car forward.
Electric car batteries work by sending electrons from the battery’s negative electrode to its positive electrode through a chemical medium called an electrolyte. The electrolyte allows ions to flow between the electrodes, completing the circuit and creating an electrical charge. The lithium-ion battery in an electric car uses lithium as its main ingredient, which is incredibly efficient at storing energy.
As technology advances, we can expect to see even more advanced batteries that are smaller, lighter, and more powerful, creating a bright future for electric car drivers.
Electrolytes and Electrodes
Electric car batteries work by converting chemical energy into electrical energy. This is made possible through the use of electrolytes and electrodes in the battery cells. When the battery is charged, a chemical reaction occurs that causes the electrodes to become positively or negatively charged.
These charged electrodes then attract ions in the electrolyte, resulting in a flow of electrons from one electrode to the other through an external circuit. This flow of electrons is what creates the electrical energy that powers the electric car. The most commonly used type of electrode in electric car batteries is made of lithium, which has a high energy density and is lightweight.
The electrolytes used in the battery can be either liquid or solid, depending on the specific technology used. Overall, the use of these materials in electric car batteries allows for efficient and clean energy storage, making them a crucial component in the growing popularity of electric vehicles.
The Role of Lithium in Lithium-ion Batteries
Lithium-ion batteries are used in various electronic devices, including electric cars. These batteries work by utilizing chemical reactions between electrodes that release energy in the form of electric current. Lithium plays an essential role in lithium-ion batteries, as it is the material used for the electrodes.
More specifically, lithium ions are transferred between positive and negative electrodes during the charging and discharging of the battery. This movement of ions creates a flow of electric current, which can power an electric motor in a car. Lithium is a lightweight and highly reactive metal, making it an ideal choice for batteries that need to be portable and efficient.
However, it can be dangerous to handle and requires careful manufacturing to prevent explosions. Despite these challenges, lithium-ion batteries are becoming more prevalent in electric vehicles due to their high energy density and ability to store large amounts of energy in a small space. With continued research, lithium-ion batteries may become even more efficient and cost-effective in the future, further advancing the development of electric cars as a reliable and sustainable mode of transportation.
Factors that Affect Electric Car Battery Life
The chemistry behind electric car batteries plays a vital role in their lifespan and overall performance. One factor that affects battery life is the type of cathode material used in the battery. Lithium-ion batteries, which are widely used in electric cars, can use a variety of cathode materials such as cobalt, nickel, and manganese.
Cobalt is known for its high energy density but can also be expensive and environmentally harmful. On the other hand, nickel and manganese are more sustainable options but may not provide the same level of performance as cobalt. Additionally, the temperature at which the battery operates also plays a significant role in its lifespan.
If the battery operates at high temperatures for extended periods, it can lead to degradation and reduced capacity. Finally, the number of charge and discharge cycles the battery undergoes also affects its lifespan. Overall, understanding the chemistry behind electric car batteries is crucial in developing longer-lasting and more sustainable energy alternatives.
Temperature and Climate
When it comes to electric cars, the temperature and climate can have a significant impact on the battery life. High temperatures can cause the battery to degrade faster due to increased chemical reaction rates, and extreme cold temperatures can also decrease the battery’s efficiency. Additionally, humidity and altitude can affect the range and performance as well.
Therefore, it is vital to keep track of the weather conditions and modify your driving accordingly to prolong the life of your car’s battery. You can also utilize various techniques and resources to regulate the temperature within the vehicle, such as preheating or pre-cooling the interior before driving, parking in shade or covered areas, and keeping the battery charged to its recommended level. All these factors contribute to the overall health and performance of an electric car, ensuring it remains in top condition while saving you money on future battery replacements and reducing your ecological footprint.
Charging and Discharging Cycles
Electric Car Battery Life Electric vehicles are becoming more popular nowadays due to their eco-friendliness, low fuel cost, and efficient performance. The electric car battery is one of the most important components that determine the overall performance of an electric vehicle. One of the major factors that affect electric car battery life is charging and discharging cycles.
Every time you charge and discharge the battery, it goes through a cycle, and these cycles can impact the life of the battery. The number of cycles the battery can handle before it degrades depends on various factors such as how you charge and discharge the battery, the temperature, and how much you use the vehicle. For instance, fast charging can reduce the battery life as it produces more heat and puts stress on the battery.
Similarly, if you frequently discharge the battery fully, it can also reduce the battery life. Therefore, it’s important to maintain a balance between charging and discharging the battery, avoiding extreme temperatures, and utilizing efficient charging methods. By doing so, you can prolong the battery life, save money on maintenance and replacements, and contribute to a cleaner environment.
New Developments in Electric Car Batteries
The chemistry behind electric car batteries is constantly advancing, leading to new developments that improve their efficiency and reliability. One promising development is the use of solid-state batteries, which use a solid electrolyte instead of a liquid one. This makes them safer, as there is no risk of leaks or fires, and also allows for higher energy densities and faster charging times.
Another new development is the use of lithium-sulfur batteries, which have a higher energy density than traditional lithium-ion batteries and are also more sustainable, as sulfur is a more abundant and affordable resource than the metals used in lithium-ion batteries. These advances in electric car battery technology are exciting, as they offer the potential for longer driving ranges and faster charging times, making electric cars a more practical and appealing option for consumers.
Solid-state Batteries and Beyond Lithium-ion Technology
Electric car batteries have come a long way in recent years, with new developments showing promise for even better performance and sustainability in the future. One area of focus is solid-state batteries, which use a solid electrolyte instead of a liquid one, making them more efficient, longer lasting, and potentially safer than traditional lithium-ion batteries. Toyota, for example, plans to release electric cars with solid-state batteries by 202
But beyond that, researchers are also exploring alternative materials for batteries, such as sodium-ion and magnesium-ion, which could offer even greater energy density and reduce dependence on limited lithium resources. As more and more electric vehicles hit the road, innovative battery technologies will be essential for driving progress in this important area of sustainable transportation.
The Future of Electric Car Batteries
Electric Car Batteries The demand for electric vehicles is increasing rapidly, and with it, the need for batteries that can power these cars for longer distances. Luckily, there are many new developments in the field of electric car batteries that are paving the way for an even brighter future. One of the most exciting developments is solid-state batteries.
These new batteries use a solid electrolyte instead of a liquid one, making them safer, more efficient, and able to store more energy in a lighter and smaller space. This technology has been around for a while, but now, companies like Toyota and BMW are investing heavily in it, with the hope of producing solid-state batteries for their vehicles by 202 Additionally, there are also new battery chemistries being developed, such as lithium-sulfur and lithium-air.
These new chemistries also have the potential to store more energy and be more affordable than the current lithium-ion batteries. With these developments, the future of electric car batteries looks brighter than ever before, and soon, we could see a world where electric vehicles are the norm.
Conclusion
In concluding the chemistry behind electric car batteries, we can say that it’s a fantastic fusion of science and technology that converts chemical reactions into clean energy. From the cathode to the anode, from lithium ions to electrons, the chemistry of these batteries is precise and smart. We can appreciate the fact that electric car batteries not only reduce our carbon footprint but also have the potential to revolutionize the automotive industry.
Therefore, as we move forward towards a cleaner and greener world, the chemistry behind electric car batteries will play a crucial role in shaping our future. So, let’s buckle up and plug in, because chemistry is the driving force of our electric dreams!”
FAQs
What is the difference between a traditional car battery and an electric car battery?
Traditional car batteries use lead-acid chemistry, while electric car batteries use lithium-ion or other advanced chemistries.
How are electric car batteries recharged?
Electric car batteries are typically charged using plug-in chargers at charging stations or using a home charging system.
What are the pros and cons of using different chemistries for electric car batteries?
Lithium-ion batteries are lighter and have a higher energy density, but they can be more expensive and have a shorter lifespan compared to other chemistries.
Can electric car batteries be recycled?
Yes, many components of electric car batteries can be recycled, including the metals and other materials used in the cells. Recycling can help reduce waste and lower the environmental impact of electric vehicles.