The Electric Revolution: Decoding the Chemistry Behind Lithium-Ion Batteries in Electric Cars
Have you ever wondered how electric cars can run for such a long time without needing a recharge? Lithium-ion batteries are the key to the long-lasting success of electric cars. These batteries function much like the rechargeable batteries we use in our small electronic devices such as phones and laptops. However, the chemistry behind lithium-ion batteries is much more complex than just plugging them into a charger.
In this blog, we will discuss the chemistry behind lithium-ion batteries and how it’s bringing a revolution to the auto industry. Let’s dive in and uncover the magic behind the success of electric cars.
What are Lithium Ion Batteries?
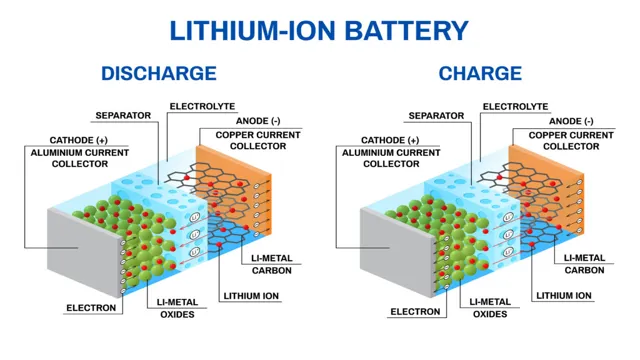
Lithium ion batteries are the primary source of power in electric cars. The chemistry behind these innovative batteries is fascinating. Essentially, the battery functions by using a lithium ion to move from the negative electrode (cathode) to the positive electrode (anode).
When the battery is charging, the lithium ions move back to the negative electrode through an electrolyte, which is usually a liquid or gel. The process repeats itself every time the battery is charged and discharged. The structure of the battery is designed to ensure that it’s safe, efficient and has a long lifespan.
One of the reasons lithium ion batteries are popular is because they are lightweight, which is advantageous for electric cars considering the weight of the battery affects the overall range of the vehicle. Overall, understanding the chemistry and engineering principles behind lithium ion batteries is beneficial for anyone interested in electric cars and modern technology.
Chemical Composition of Lithium Ion Batteries
Lithium ion batteries are the most commonly used rechargeable batteries in electronic devices such as cell phones, laptops, and electric vehicles. They are known for their high energy density and long cycle life. The chemical composition of lithium ion batteries includes a negative electrode made of graphite, a positive electrode made of a lithium metal oxide, and an electrolyte made of a lithium salt in an organic solvent.
When the battery is charged, lithium ions move from the positive electrode to the negative electrode through the electrolyte and create electrical energy. This process is reversed when the battery is discharged. Understanding the chemical composition of lithium ion batteries is important for ensuring their safe and efficient use.
With the increasing demand for renewable energy sources, lithium ion batteries will continue to play a key role in powering our modern world.

How Do Lithium Ion Batteries Work?
Lithium ion batteries are ubiquitous energy storage devices that power countless portable electronic devices, electric cars, and even some houses. These batteries work by transferring lithium ions between two electrodes, one of which contains lithium-based compounds and the other uses carbon-based materials. When the battery is connected to a device, lithium ions flow from the anode to the cathode, producing electrical energy that powers the device.
When the battery is charged, the flow is reversed, with lithium ions moving back to the anode. The materials used in lithium ion batteries are carefully chosen to maximize energy density, safety, and efficiency. While lithium ion batteries have some limitations, such as capacity degradation and the potential for overheating or even catching fire, their benefits far outweigh the drawbacks.
These batteries provide a convenient, reliable, and cost-effective way to store and use electrical energy, and their use in various applications is projected to continue to grow.
Advantages of Lithium Ion Batteries in Electric Cars
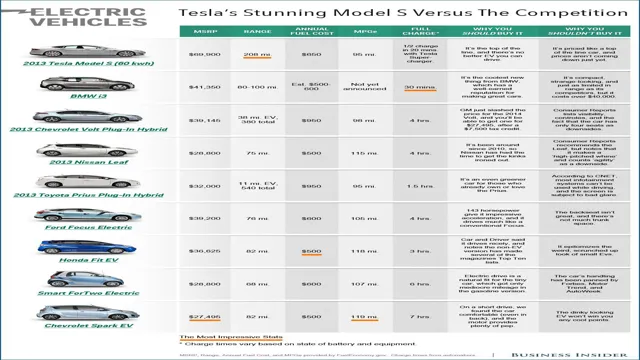
The chemistry behind lithium ion batteries in electric cars is the key to unlocking the many advantages that these vehicles offer. Lithium ion batteries are made up of an anode, cathode, and an electrolyte, which work together to produce electric currents. Compared to traditional lead-acid batteries, lithium ion batteries have a higher energy density and can store more power.
This means that electric cars with lithium ion batteries can travel longer distances on a single charge. Lithium ion batteries are also much lighter than lead-acid batteries, which means they can provide more power while taking up less space. Furthermore, lithium ion batteries have a longer lifespan and can be recharged faster than traditional batteries.
All of these advantages make lithium ion batteries the preferred choice for electric cars and the driving force behind their impressive performance.
Energy Density
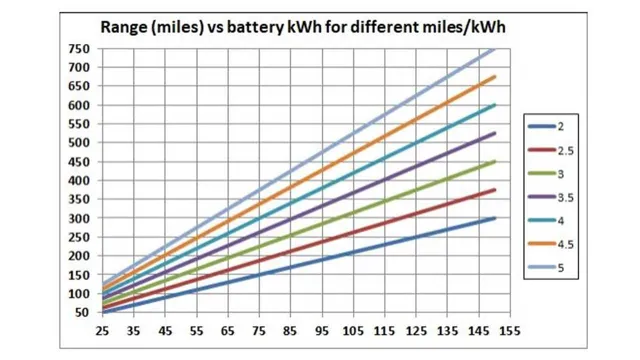
The high energy density of lithium-ion batteries makes them the perfect choice for electric cars. Energy density measures the amount of energy stored in a battery per unit of volume or weight, and it’s crucial for electric cars because they need to be as lightweight as possible to maximize their range. Lithium-ion batteries have the highest energy density of any rechargeable battery, which means that they can store more energy in a smaller space.
This makes them ideal for use in electric cars because it allows for a longer range without compromising on weight or performance. Additionally, lithium-ion batteries are more efficient, durable, and have a longer lifespan compared to traditional conventional batteries. This is why most of the electric car manufacturers utilize lithium-ion batteries as their primary power source.
Efficiency and Durability
When it comes to electric cars, the advantages of lithium ion batteries are hard to ignore. These batteries are highly efficient, meaning that they can store a lot of energy in a small space. This makes them perfect for use in electric vehicles, where space is at a premium.
Additionally, lithium ion batteries are durable, meaning that they can withstand a lot of use without degrading too quickly. This is important in the context of electric cars, which need a battery that can last for hundreds of thousands of miles. Compared to other types of batteries, lithium ion batteries are also less prone to failure, which means that they are less likely to leave you stranded on the side of the road.
Overall, the advantages of lithium ion batteries make them a very attractive option for those who are looking to go electric.
Safety Features
Lithium Ion Batteries Electric cars are revolutionizing the way we think about transportation, and one of the technologies that make them possible is the lithium-ion battery. While there are still challenges to be addressed, such as the limited range of these batteries, they come with several advantages that improve the safety, performance, and sustainability of electric vehicles. For one thing, lithium-ion batteries are less prone to catching fire than other types of batteries, which makes them a safer option for use in cars.
They also have a higher energy density, which means they can store more energy in a smaller space, giving electric cars a longer range and better acceleration. They are also more efficient, with less charge loss and longer life cycles, reducing the need for frequent replacements. Additionally, since lithium-ion batteries do not require hazardous materials like lead-acid or nickel-metal hydride batteries, they are more environmentally friendly and easier to recycle.
All these factors make lithium-ion batteries a compelling option for powering the next generation of electric cars.
Challenges and Limitations of Lithium Ion Batteries in Electric Cars
The chemistry behind lithium ion batteries in electric cars plays a crucial role in driving the shift towards sustainable transportation. However, these batteries still face some challenges and limitations. One limitation is the high cost of producing the batteries due to the scarcity of lithium, cobalt and other rare earth elements.
Another challenge is the limited range of electric cars due to the battery’s energy density and weight. As a result, car manufacturers are looking for ways to improve the battery’s chemistry to increase its efficiency, energy density, and lifespan. Some promising technologies include solid-state batteries and lithium-air batteries that can increase energy density while reducing weight.
Nonetheless, more research is required to overcome these limitations and make electric cars more affordable and practical for the masses. Ultimately, the chemistry behind lithium ion batteries in electric cars will play a significant role in shaping the future of transportation as we move towards a more sustainable and eco-friendly society.
Cost
The cost of lithium-ion batteries is one of the major challenges faced by the electric car industry. These batteries are expensive to produce, which makes electric cars more expensive to manufacture. Additionally, lithium-ion batteries have a limited lifespan, and they need to be replaced periodically, which adds to the cost.
However, advancements in technology have led to a significant reduction in the cost of lithium-ion batteries over the years. With further research and development, it is possible to bring down the cost even more. Furthermore, some manufacturers are exploring alternative battery chemistries that could potentially offer better performance at a lower cost.
Despite the challenges and limitations of lithium-ion batteries, they remain the most viable option for powering electric cars at present.
Environmental Impact
The rise of electric cars is considered a sustainable solution towards reducing our carbon footprint. However, the production and disposal of lithium-ion batteries used in electric cars come with their own set of environmental challenges and limitations. One of the primary concerns is the sourcing of raw materials used to make the batteries.
The extraction of lithium, cobalt, and other rare earth minerals required for battery production is a resource-intensive process that can have detrimental effects on the environment. Additionally, the manufacturing process itself requires massive amounts of energy, which is often derived from non-renewable sources. Furthermore, the disposal of lithium-ion batteries presents a significant challenge.
These batteries contain toxic and hazardous materials, which can cause extensive environmental damage if not disposed of properly. Disposing of these batteries also presents a potential safety hazard for workers dealing with the hazardous waste. To address these challenges and limitations, initiatives are being taken to develop alternative battery technologies that are more sustainable and less resource-intensive.
However, until sustainable alternatives are developed, it is crucial to minimize the environmental impact of electric car batteries by promoting efficient recycling and disposal practices. Only by actively tackling these issues can we truly make electric cars a sustainable alternative for the future.
Future of Lithium Ion Batteries in Electric Cars
The chemistry behind lithium ion batteries in electric cars is what makes them stand out from other types of batteries. Lithium ion batteries are made up of positively charged lithium ions that move from the negative electrode to the positive electrode through an electrolyte. The electrodes themselves are made up of a host of different materials, including graphite and other metals such as cobalt or nickel.
The chemistry behind these batteries is constantly evolving and improving, as scientists and researchers look to make them more efficient and longer-lasting. Some of the most exciting advancements in the chemistry of lithium ion batteries include the development of solid-state batteries, which could offer even higher energy densities and safer operation than traditional lithium ion batteries. As electric cars continue to grow in popularity, the chemistry behind their power sources will undoubtedly continue to evolve and improve, leading to even better performance and sustainability.
With continued advancements in the field, the future of lithium ion batteries in electric cars is looking brighter than ever.
Conclusion
In conclusion, the chemistry behind lithium-ion batteries in electric cars is truly electrifying! With the unique ability to store and release energy efficiently, these batteries have revolutionized the automotive industry and paved the way for a cleaner, greener future. So next time you’re cruising down the highway in your electric vehicle, take a moment to appreciate the amazing chemistry at work that makes it all possible.”
FAQs
What is a lithium ion battery and how does it work in electric cars?
A lithium ion battery is a type of rechargeable battery that uses lithium ions to store and release energy. In electric cars, these batteries are used to power the vehicle’s electric motor.
How are lithium ion batteries different from traditional lead-acid batteries in cars?
Lithium ion batteries are lighter and more compact compared to traditional lead-acid batteries. They also have a higher power density, which means they can store more energy in a smaller space.
What are the advantages of using lithium ion batteries in electric cars?
Lithium ion batteries have a longer lifespan compared to traditional batteries. They also have a higher energy density, which means they can provide more power and last longer on a single charge.
What is the chemistry behind lithium ion batteries and how does it affect their performance in electric cars?
Lithium ion batteries use a combination of lithium cobalt oxide and graphite to store and release energy. The chemistry behind these batteries affects their performance by determining their energy density and lifespan. Continuous research is being done to find ways to improve the chemistry of these batteries.