The Science Behind Electric Car Battery: Exploring the Chemistry of Sustainable Driving
Electric cars are the future of the automobile industry. They offer numerous benefits to both the environment and drivers. But have you ever wondered about the chemistry behind the battery that powers these vehicles? What makes them so special and how do they work? In this blog post, we will explore the fascinating chemistry behind the electric car battery and shed light on how it works.
From the anode to the cathode and everything in between, we will dive deep into this groundbreaking technology and reveal the science that makes it all possible. So buckle up and get ready for a ride into the world of electric car batteries!
Introduction
When it comes to electric cars, one of the most important components is the battery. The battery in an electric car is responsible for powering the vehicle, and as such, it is crucial that the battery is of high quality and durability. The chemistry of electric car batteries is complex, and there are several factors that determine how well a battery performs.
The most common type of battery used in electric cars is a lithium-ion battery, which has high energy density and is relatively lightweight. The battery is made up of several layers, including the positive and negative electrodes, the electrolyte, and the separator. The chemistry of the battery determines how it charges and discharges, and how efficiently it converts energy.
By understanding the chemistry of electric car batteries, we can continue to improve the technology and make electric cars more efficient and sustainable for the future.
Overview of Electric Car Battery Chemistry
Electric Car Battery Chemistry Electric car battery chemistry is the driving force behind the increasing popularity of electric vehicles. These batteries are the heart of the electric vehicle, storing and providing the energy required to power the car. Whether you own or plan to purchase an electric vehicle, understanding the battery’s chemistry is crucial.
Some of the most common battery types used in electric cars are lithium-ion, nickel-metal hydride, and lead-acid batteries. Each battery chemistry has its unique characteristics and performance, affecting the electric vehicle’s driving range and charge rate. Knowing the basics of electric car battery chemistry can help you make more informed decisions when buying, charging, and maintaining your electric vehicle.
Composition of Battery Cells
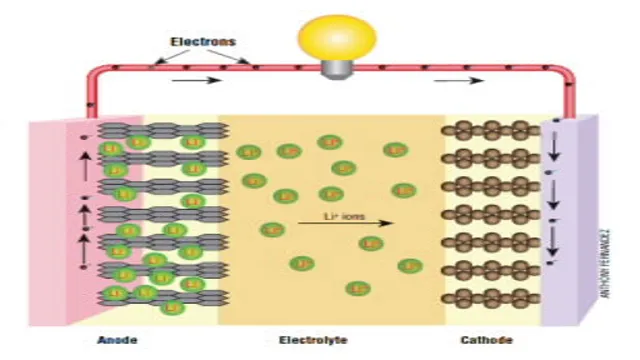
The chemistry of electric car batteries involves the composition of battery cells and the materials used to create them. Lithium-ion batteries, which are commonly used in electric cars, consist of positive and negative electrodes made from lithium compounds, such as lithium cobalt oxide or lithium iron phosphate, and an electrolyte, typically a lithium salt dissolved in an organic solvent. When a current is applied, lithium ions move from the negative electrode to the positive electrode through the electrolyte, creating an electrical charge that can power the car.
To enhance the efficiency and durability of electric car batteries, researchers are exploring new materials and designs to improve the conductivity and stability of the electrodes and electrolytes. The chemistry of electric car batteries is continuously evolving, and with advancements in technology, we can expect more efficient, longer-lasting, and sustainable batteries to power our cars in the future.
Cathode, Anode, and Electrolyte Components
Battery cells are made up of three components: cathode, anode, and electrolyte. The cathode is the positive electrode, while the anode is the negative electrode. The electrolyte is the solution that connects the cathode and anode and allows the flow of ions between them.
These three components work together to create the chemical reaction that produces electricity in the battery. The cathode and anode are typically made of different materials such as metal oxides, carbon, or lithium. The electrolyte solution can vary depending on the type of battery, but it is usually made of a salt or acid dissolved in a solvent.
Understanding the composition of battery cells is essential to determine their capacity, voltage, and overall performance. Different combinations of cathode, anode, and electrolyte can lead to varying properties, such as energy density, power density, and safety. The chemistry of battery cells is complex, but its practical application is ubiquitous in our daily lives, powering everything from smartphones and electric vehicles to large-scale energy storage systems.
Types of Materials Used in Battery Cells
Battery cells are composed of various materials that work together to generate and store electrical energy. The most common components of battery cells are the cathode, anode, electrolyte, and separator. The cathode is usually made of metal oxides, such as lithium cobalt oxide or lithium manganese oxide, while the anode is typically made of graphite or other carbon-based materials.
The electrolyte is a solution or gel that facilitates the movement of ions between the cathode and anode, while the separator prevents the two from touching and short circuiting. Some newer battery technologies use different materials, such as solid-state electrolytes or organic materials. Regardless of the specific materials used, the composition of battery cells plays a crucial role in their performance, lifespan, and safety.
By understanding how different materials interact, researchers can continue to improve battery technology and develop more efficient and sustainable energy storage solutions.
Battery Chemistry and Performance
If you’re curious about the chemistry of electric car batteries, you’re not alone. These batteries are essential to powering a vehicle that runs solely on electric power, and their performance is closely tied to their chemical makeup. Most electric car batteries are made up of lithium-ion cells, which use an electrolyte solution to transport ions between the positive and negative electrodes.
The specific composition of the electrolyte solution can vary depending on the manufacturer, and plays a role in determining the battery’s energy density and overall performance. Additionally, the electrodes themselves are typically made of different materials, such as lithium cobalt oxide, lithium nickel manganese cobalt oxide, or lithium iron phosphate, which can impact the battery’s capacity, durability, and cost. Factors like temperature and usage patterns can also affect the lifespan of the battery, making it important for electric car owners to stay informed and take good care of their vehicle’s battery.
Overall, the chemistry of electric car batteries is a complex but fascinating subject with important implications for the future of clean transportation.
Impact of Battery Chemistry on Energy Density
Energy density When it comes to batteries, their chemistry plays a crucial role in determining their performance, and most importantly, their energy density. Energy density refers to the amount of energy that can be stored in a battery per unit of volume or weight. Various battery chemistries exhibit different energy densities, and this can have a significant impact on the battery’s overall performance.
For instance, lithium-ion batteries have a high energy density, which makes them an ideal choice for portable devices like smartphones and laptops. On the other hand, lead-acid batteries have a lower energy density but are commonly used in cars and other vehicles. Ultimately, battery manufacturers carefully select their chemistry based on the application and desired performance, balancing energy density, cost, and safety.
Effect of Operating Temperature and Voltage
When it comes to batteries, performance can be greatly affected by both operating temperature and voltage. Different battery chemistries also react differently to changes in these factors. For example, lithium-ion batteries, the type commonly found in electronic devices, can lose capacity and suffer damage when exposed to high temperatures.
On the other hand, lead-acid batteries, typically used in vehicles, perform better at higher temperatures. Additionally, both high and low voltages can have negative impacts on battery health. Too high can lead to overheating and potential explosions, while too low can cause battery damage and reduce capacity.
It’s important to keep both operating temperature and voltage within safe ranges to ensure optimal battery performance and longevity.
Recent Advances in Battery Chemistry
The chemistry of electric car batteries has been an area of intense research and development over the past few years, leading to some truly exciting advances. One of the most promising developments is the use of solid-state battery technology, which replaces the traditional liquid electrolyte with a solid material. This has the potential to greatly increase the capacity of batteries, while also improving safety and reducing the risk of fire.
Another area of research has focused on increasing the energy density of batteries, which would allow them to store more power in a smaller form factor. This could lead to electric vehicles with longer ranges and smaller, lighter batteries. Overall, the future of electric car batteries looks very bright, and the advances being made in battery chemistry are likely to play a key role in the transition to a more sustainable, low-carbon future.
Introduction of Novel Materials
Battery technology has seen a significant shift recently, with researchers focusing on developing novel materials that can improve its performance. One area of focus has been battery chemistry. Recent advances in battery chemistry have led to the discovery of new materials, such as metal-air batteries, which have the potential to revolutionize the industry.
These batteries use oxygen as a reactant, making them much lighter than traditional batteries. Additionally, scientists have started exploring the use of solid-state electrolytes to replace traditional liquid electrolytes. This is because solid-state electrolytes offer several benefits, including better safety and stability, longer shelf life, and higher energy density.
One of the recent breakthroughs in battery chemistry is the development of Lithium-sulfur (Li/S) batteries. Li/S batteries are touted to have a higher energy density, lower cost, and a longer lifespan than conventional Lithium-ion (Li-ion) batteries. They are also more environmentally friendly as they use sulfur, which is abundantly available and cheaper than the materials used in Li-ion batteries.
This makes them an attractive alternative for storing renewable energy. These novel materials have the potential to dramatically improve the efficiency and performance of batteries used in various applications, ranging from electric vehicles and portable electronics to grid-scale energy storage systems. While challenges, such as scalability and cost, remain, continued research into battery chemistry is expected to yield many more exciting discoveries in the years to come.
Innovative Electrolyte Solutions
Innovative Electrolyte Solutions There have been many recent advances in battery chemistry that show promising results for improving the efficiency of lithium-ion batteries. A key area of focus has been on finding new and innovative electrolyte solutions to replace the standard liquid electrolytes used in these batteries. Solid-state electrolytes have gained a lot of attention in recent years due to their potential to improve safety and increase energy density.
These electrolytes are composed of solid materials that can conduct ions, such as lithium, much like their liquid counterparts. However, solid-state electrolytes have several advantages over liquid electrolytes. They are less volatile and can operate at higher temperatures, which could eliminate the need for complex cooling systems in high-performance applications.
Moreover, there have been successful experiments with using polymer electrolytes in batteries, which are made from soft, flexible, and lightweight polymers that could lead to lighter and more flexible batteries for wearables and portable electronics. As we continue to develop more advanced materials and technologies, it’s exciting to see the potential for improving energy storage and increasing the lifespan of batteries.
Conclusion
In conclusion, electric car batteries may seem like magical devices, but they are actually the result of careful and precise chemistry. Through a complex interplay of chemistry and engineering, electric car batteries are able to store and release energy with impressive efficiency and reliability. So the next time you hop into your electric car and zoom down the highway, remember that you’re not just driving a marvel of modern technology – you’re also witnessing the amazing power of chemistry in action.
“
FAQs
What is the chemistry behind electric car batteries?
Electric car batteries typically use lithium-ion chemistry, which involves the movement of lithium ions between electrodes to store and release energy.
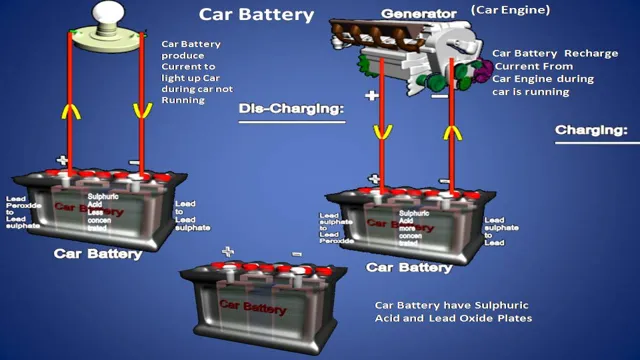
How do electric car batteries differ from traditional car batteries?
Traditional car batteries use lead-acid chemistry, while electric car batteries use lithium-ion chemistry. Electric car batteries also have a higher energy density and can store more energy.
What are the advantages of using lithium-ion chemistry in electric car batteries?
Lithium-ion chemistry allows for higher energy density, faster charging times, and longer battery life compared to traditional lead-acid chemistry.
Are there any environmental concerns associated with the chemistry of electric car batteries?
While lithium-ion chemistry is generally considered more environmentally friendly than traditional lead-acid chemistry, there are concerns about the extraction and disposal of the materials used in lithium-ion batteries. Additionally, the production of electric car batteries still requires a significant amount of energy and resources.