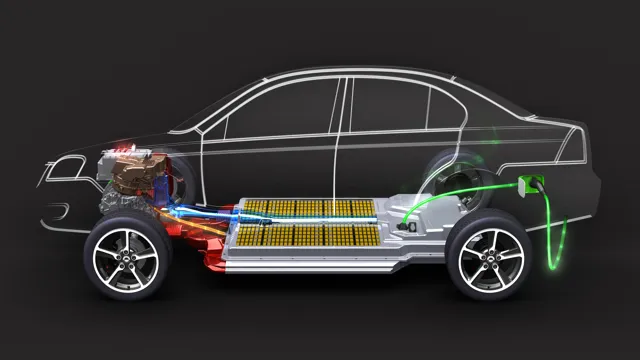
The Shocking Truth Behind Electric Car Batteries – What Are They Really Made Of?
If you’re interested in electric cars, you might have wondered what makes them run. After all, a car that runs on electricity instead of gas requires a different power source altogether. The answer is simple battery power.
However, the question then becomes, what are electric car batteries made of? These batteries are a crucial component of electric cars, and yet many people don’t know the answers to these questions. In this blog, we’ll explore what electric car batteries are made of, demystifying the technology behind it. So, buckle up and let’s dive in!
Lithium-ion Batteries
Electric car batteries are usually made of lithium-ion technology, which has become the industry standard. Lithium-ion batteries are in high demand because of their high energy density, low self-discharge rates, long cycle life, and relatively low maintenance requirements. These batteries combine both power and energy, making them ideal for electric vehicles, where high-energy density allows for extended driving ranges, and high power output provides quick acceleration.
Lithium-ion batteries consist of positive and negative electrodes separated by an electrolyte. When charged, lithium ions move from the positive electrode through the electrolyte to the negative electrode, where they are stored until needed. When discharged, the reverse charge causes the lithium ions to move back through the electrolyte to the positive electrode, releasing energy in the process.
In summary, lithium-ion batteries are essential components of electric cars, providing power and range, and have become the industry standard due to their high performance and reliability.
Composition of Lithium-ion Batteries
Lithium-ion batteries are the most common rechargeable batteries used in electronic devices. They work by transferring ions between a cathode (positive electrode) and an anode (negative electrode) through an electrolyte. The cathode is typically made of lithium metal oxide, while the anode is made of graphite.
The electrolyte is usually a lithium salt in an organic solvent. When the battery is charged, lithium ions are extracted from the cathode and move through the electrolyte to the anode, where they are stored. When the battery is discharged, the process is reversed, with the lithium ions moving back to the cathode.
The efficiency and lifespan of lithium-ion batteries depend on the quality and composition of their materials, as well as their design and usage. With their high energy density and low self-discharge rate, lithium-ion batteries have become essential components in portable electronic devices, electric vehicles, and renewable energy systems.

Advantages of Lithium-ion Batteries
Lithium-ion batteries are becoming increasingly popular due to their many advantages over other types of batteries. One of the main advantages is their high energy density, which means they can store more energy in the same volume or weight as other batteries. This makes them ideal for use in portable electronic devices, such as smartphones and laptops, which require a long battery life but don’t have much space for a large battery.
Another advantage is their low self-discharge rate, which means they can hold their charge for longer periods of time without needing to be recharged. Lithium-ion batteries are also more environmentally friendly than other types of batteries, as they do not contain toxic metals such as lead or mercury. Finally, they are more efficient than other battery types, and can be recharged many times without losing their capacity.
Overall, the advantages of lithium-ion batteries make them an excellent choice for a wide range of applications, from consumer electronics to electric vehicles.
Nickel-Metal Hydride Batteries
Electric car batteries are typically made up of a combination of materials, but one type of battery commonly used is the nickel-metal hydride battery. These batteries are rechargeable and typically weigh less than their lead-acid counterparts, making them more suitable for use in electric vehicles. Nickel-metal hydride batteries have a high energy density, which means they can store a lot of power in a relatively small volume.
This makes them the perfect battery choice for electric vehicles, as a lot of power needs to be stored to power the car’s electric motor. Additionally, nickel-metal hydride batteries have a long lifespan, meaning they’re a more sustainable choice for electric vehicles. Though newer battery technologies are emerging, nickel-metal hydride batteries still play a hefty role in powering the electric car industry today.
Composition of Nickel-Metal Hydride Batteries
Nickel-Metal Hydride Batteries Nickel-metal hydride batteries, or NiMH batteries, are rechargeable batteries commonly used in electronic devices such as cameras, toys, and cordless phones. These batteries consist of a positive electrode made of nickel hydroxide and a negative electrode made of hydrogen-absorbing alloy. The electrolyte used in NiMH batteries is typically potassium hydroxide.
The composition of NiMH batteries allows for a higher energy density than traditional nickel-cadmium batteries, meaning they can store more energy in a smaller size. Additionally, they are more environmentally friendly and do not contain toxic chemicals like cadmium. NiMH batteries also have a longer lifespan and are less prone to the “memory effect” that can affect other types of rechargeable batteries.
However, there are some disadvantages to NiMH batteries. They can be more expensive than other types of batteries, and they are not as efficient in extreme temperatures. They also have a slightly lower energy density than lithium-ion batteries, which are commonly used in smartphones and other portable devices.
Overall, NiMH batteries are a reliable and eco-friendly choice for many everyday electronic devices. They offer a balance of efficiency and environmental consciousness, making them a popular alternative to traditional batteries.
Advantages of Nickel-Metal Hydride Batteries
Nickel-Metal Hydride batteries are a popular choice for many electronic gadgets and vehicles due to their many advantages. One benefit is that they have a higher energy density than other rechargeable batteries, meaning that they can hold more charge and last longer. This makes them ideal for devices that require a lot of power, such as electric vehicles, laptops, and power tools.
Additionally, Nickel-Metal Hydride batteries are environmentally friendly and less toxic than other battery types, making them a better choice for the planet. They also have a longer lifespan than most other rechargeable batteries, which means fewer replacements and less waste. Overall, Nickel-Metal Hydride batteries are a reliable and efficient energy source that provides many benefits for both consumers and the environment.
Lead-Acid Batteries
If you are wondering what electric car batteries are made of, then you might be surprised to learn that lead-acid batteries are commonly used. These batteries have been around for almost 160 years and are still the most well-known type of rechargeable battery. They consist of lead plates submerged in an acid electrolyte solution, which converts chemical energy into electrical energy.
Lead-acid batteries have a low cost and high energy density, which makes them perfect for use in electric cars. However, they are quite heavy and bulky, which can pose a challenge for car manufacturers who are trying to design more lightweight and efficient vehicles. As the demand for electric cars continues to grow, there is a push to develop new and more advanced battery technologies that can address these issues.
Nonetheless, lead-acid batteries continue to play an important role in powering electric cars and other automotive applications.
Composition of Lead-Acid Batteries
Lead-acid batteries are the most commonly used batteries in automobiles, and they are also used as backup power supplies for computer systems and other electronics. The composition of these batteries consists of several parts, including the positive and negative plates, the separators, the electrolyte, and the casing. The plates are made of lead, while the separators are made of a porous material, such as rubber or plastic.
The electrolyte is a solution of sulfuric acid and water, which facilitates the flow of electricity between the plates. The casing is typically made of hard plastic to protect the internal components from damage, and is often sealed to prevent leaks. These components work together to create a reliable and efficient power supply, making lead-acid batteries a popular choice for a wide range of applications.
Advantages of Lead-Acid Batteries
Lead-acid batteries have been around for over a century, and they remain one of the most widely used types of batteries today. Their popularity is due to their numerous advantages. Firstly, they are durable and can withstand repeated deep cycling.
Secondly, they are relatively inexpensive, making them a popular choice for many applications. Thirdly, they are easy to recycle, making them an environmentally friendly option. Fourthly, they can deliver high bursts of power, making them ideal for starting large engines or powering heavy machinery.
Lastly, they require very little maintenance, making them an easy choice for people who don’t have the time or resources to constantly monitor their batteries. These are just a few of the many advantages that lead-acid batteries have to offer, making them a reliable and effective choice for a wide range of applications.
Conclusion
In conclusion, electric car batteries are made up of a combination of lithium, cobalt, nickel, and other materials that are carefully engineered to provide maximum performance and longevity. Think of these batteries like a fine wine, with each ingredient contributing to the overall flavor and character – but instead of a delightful buzz, you get a smooth, silent ride. So next time you hop into your electric vehicle, take a moment to appreciate the incredible technology that powers your ride and the careful craftsmanship that went into creating its battery.
“
FAQs
What materials are used to make electric car batteries?
Electric car batteries are typically made of lithium-ion cells, which contain lithium, carbon, and oxygen.
Can electric car batteries be recycled?
Yes, electric car batteries can be recycled. The materials inside the battery, such as lithium, cobalt, nickel, and copper, can be extracted and reused.
How long do electric car batteries last?
The lifespan of electric car batteries can vary depending on factors such as usage, temperature, and charging habits. On average, electric car batteries can last for several years before needing to be replaced.
Are electric car batteries safe?
Yes, electric car batteries are designed to be safe and undergo rigorous testing to ensure their safety. They also have built-in safety features, such as thermal management and overcharge protection.