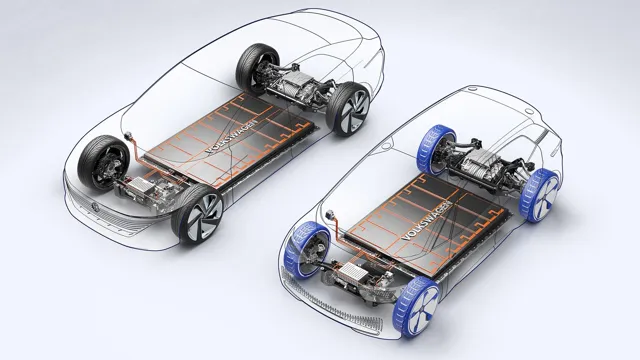
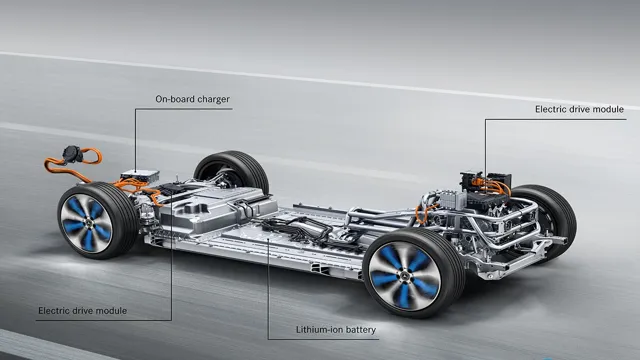
Revolutionizing the Automotive Industry: Exploring the Components of Electric Car Battery Cells
Electric cars have been gaining popularity over the years as more and more people seek eco-friendly transportation options. Battery cells are one of the most essential parts of an electric car, and their components play a crucial role in the overall performance and longevity of the battery. From the cathode and anode to the electrolyte and separator, understanding the different components that make up electric car battery cells is important for both car manufacturers and drivers alike.
In this blog, we’ll take a closer look at each of these components and explore their functions, so you can gain a better understanding of how electric car battery cells work and what makes them different from traditional combustion engines. So, buckle up and get ready to learn more about the inner workings of these eco-friendly vehicles!
An Overview of Electric Car Batteries
Electric car batteries consist of several components, including battery cells, pack, and management system. The battery cells are the primary building blocks of electric car batteries and contain a positive cathode, a negative anode, and an electrolyte. The cathode and anode store and release lithium ions during charging and discharging.
The pack is made up of multiple battery cells that are connected in series and parallel to provide the required voltage and energy. The management system oversees the charging and discharging process, monitors the temperature and health of the battery, and protects it from overheating or overcharging. One of the critical components of the electric car battery is the cell, which plays a crucial role in determining the battery’s performance and capacity.
The quality of the cell’s materials, such as the cathode and anode, can affect the range, power, and lifespan of the battery. Therefore, electric car manufacturers invest heavily in research and development to improve the quality and efficiency of the battery cell components.
The Cells that Power Electric Cars
Electric Car Batteries Electric car batteries are the heart of any electric vehicle, powering the car’s electric motor. The most common type of battery used in electric cars is the lithium-ion battery, which stores energy by moving lithium ions through a series of electrodes. These batteries are highly efficient and offer the best balance between energy density, weight, and cost.
However, they also come with certain limitations, such as a finite number of charge and discharge cycles. This means that over time, the battery’s capacity will gradually decrease, requiring replacement after a certain number of years or miles driven. To maximize the lifespan of these batteries, manufacturers use advanced battery management systems that monitor the battery’s temperature and charge levels to ensure optimal performance and prevent damage.
Overall, electric car batteries are a crucial component of any electric vehicle, and as technology continues to advance, we can expect to see even better and more efficient battery options in the years to come.

What are the Major Components of Electric Car Battery Cells?
Electric Car Batteries Electric cars are becoming more popular nowadays, and one of the most crucial components of these vehicles is the battery cells. These cells are responsible for storing and releasing the electrical energy that powers the motor. The major components of electric car battery cells include the cathode, anode, electrolyte, and separator.
The cathode is the positive electrode, while the anode is the negative electrode. The electrolyte enables the flow of charged particles between the cathode and anode, while the separator keeps them from coming in contact. The material used in making the components also plays a role in the overall efficiency and performance of the battery cells.
For instance, some of the most commonly used cathode materials include nickel, manganese, and cobalt. Additionally, the arrangement of the cells also influences the battery’s performance, with series connections providing higher voltages while parallel connections provide higher currents. These components and factors all contribute to the efficiency and longevity of electric car batteries.
Understanding Battery Cell Chemistry
When it comes to understanding electric car battery cell components, it’s important to know the basic chemistry behind them. These batteries typically consist of three main components: an anode, a cathode, and an electrolyte. The anode is made up of a material that can store lithium ions, usually graphite.
The cathode, on the other hand, is made up of a material that can accept lithium ions, such as a layered oxide. The electrolyte is the medium that allows lithium ions to flow between the anode and cathode, typically a liquid or gel substance. When a battery is charged, lithium ions are drawn from the cathode to the anode through the electrolyte, and when it’s discharged, they flow from the anode back to the cathode.
Understanding this chemical process is key to optimizing battery performance and lifespan, which will be crucial as electric cars become more widespread.
How do Lithium-Ion Battery Cells Work?
Lithium-ion batteries are a vital power source for many electronic devices such as cell phones, laptops, and electric vehicles. People often talk about these batteries, but few actually understand their chemistry. The lithium-ion battery cell functions by storing energy through a chemical reaction between the anode and the cathode.
Lithium-ion battery cells use a lithium salt electrolyte between the anode and the cathode to conduct electricity. During use, lithium ions move towards the cathode while electrons move towards the anode, resulting in a flow of energy. The flow of energy reverses when the battery is charging, where lithium ions move back towards the anode and electrons towards the cathode.
This continuous flow of lithium ions and electrons generates power. With the growing demand for portable electronic devices, the importance of understanding the chemistry behind lithium-ion batteries is more essential than ever.
The Importance of Electrolytes in Battery Cells
Electrolytes in Battery Cells When it comes to battery cell chemistry, electrolytes play an important role in the functioning of the battery cell. They are responsible for carrying electrons between the electrodes and allowing the flow of current, which powers our electronic devices. Without electrolytes, the battery cell wouldn’t be able to store or discharge energy.
Electrolytes are a type of chemical compound that can conduct electricity when dissolved in water or other solvents. In battery cells, they usually consist of a salt dissolved in a liquid or gel. The most commonly used electrolyte in rechargeable batteries is Lithium-ion electrolyte, which has excellent conductivity and stability.
The choice of electrolyte greatly affects the battery cell’s performance, safety, and cost. Some electrolytes can cause overheating, short circuits, and even explosions if they react with other materials in the battery cell. Therefore, selecting the right electrolyte is crucial for manufacturing high-quality and safe batteries.
In conclusion, understanding the importance of electrolytes in battery cells is vital for developing advanced and efficient batteries for various applications. Electrolytes play a significant role in enabling the flow of current and energy storage. By choosing the right electrolyte, it’s possible to manufacture high-performance and safe batteries that meet the increasing demands of portable electronics, electric vehicles, and renewable energy technologies.
Why Cathodes and Anodes are Critical Components of Battery Cells
Battery Cell Chemistry When it comes to battery cells, the cathode and anode are two crucial components that determine a battery’s overall performance. The cathode is the electrode where positively charged ions flow towards, while the anode is the electrode where negatively charged ions flow towards. Understanding the chemistry behind them is key to developing efficient batteries.
The cathode and anode determine a battery’s voltage, capacity, and energy density. The choice of materials used in each electrode can affect these factors dramatically, making it essential to carefully consider their selection during battery production. For example, a lithium-ion battery commonly uses graphite in the anode and lithium cobalt oxide in the cathode, while a lithium-sulfur battery uses sulfur in the cathode and a carbon-based material in the anode.
Each material combination yields unique behavior in terms of voltage, capacity, and energy density, and as such is critical to battery performance. Therefore, understanding battery cell chemistry plays an essential role in optimizing battery performance and increasing efficiency.
Charging and Discharging Electric Car Batteries
Electric car battery cell components play a crucial role in charging and discharging the battery. Each cell is made up of several components such as the cathode, anode, electrolyte, and separator. During charging, the electric current flows from the cathode through the electrolyte and into the anode, causing the lithium ions to move from the cathode to the anode.
This process is reversed during the discharging phase, where the lithium ions move from the anode back to the cathode, providing power to the car. The efficiency of this process is affected by the cell components, and the quality of the materials used in manufacturing them. The proper charging and discharging of the battery can also impact the lifespan of the battery.
Consequently, it is essential to ensure that the battery is charged and discharged correctly, as it can affect not only the car’s performance but also its safety.
The Science Behind Charging Electric Car Batteries
Electric car owners are no strangers to the science behind charging their car’s batteries. When an electric vehicle is connected to a charging station, the process of charging and discharging the battery begins. Charging involves sending an electric current to the battery, which causes the lithium ions within it to migrate from the positive electrode to the negative electrode.
As the battery charges, the voltage of the battery rises until it reaches its maximum charge capacity. Once fully charged, the electric energy stored within it can power the car. During discharging, the opposite happens.
The lithium ions move from the negative electrode to the positive electrode, creating an electric current that powers the car. However, the rate at which the battery discharges depends on the car’s usage and driving conditions. Factors such as acceleration, braking, and terrain can all influence the speed at which the battery drains.
As electric cars continue to grow in popularity, understanding the science behind charging and discharging their batteries will be essential for drivers to maximize the efficiency of their vehicles.
Why Discharging Electric Car Batteries is Different Than Traditional Batteries
When it comes to electric car batteries, charging and discharging require a different approach compared to traditional batteries. Unlike a typical car battery that can be fully discharged before recharging, electric car batteries need to be managed differently. In fact, it’s recommended to never fully discharge an electric car battery as it can cause long-term damage to the battery’s health and performance.
Instead, electric car owners should aim to keep the battery’s charge level between 20% to 80% capacity, which can help extend the battery’s lifespan and overall performance. To put it simply, discharging an electric car battery is like eating a meal – it’s better to eat several small meals throughout the day rather than one big meal. By keeping a smaller charge level and frequently “topping off” the battery, electric car owners can ensure their vehicle will keep running efficiently for years to come.
How to Extend the Life of Electric Car Battery Cells
Electric car battery cell components are essential to understand if you’re looking to extend the life of your electric vehicle’s battery. One of the best ways to do this is to keep your battery charged between 20% and 80%. Charging your battery beyond 80% and letting it drain below 20% can put a significant amount of stress on the cells, reducing their lifespan.
Additionally, it’s important to avoid exposing your battery to extreme temperatures. Heat can cause cells to degrade quickly, while cold temperatures can reduce their capacity. It’s also crucial to maintain your car’s battery health by avoiding frequent fast charging and keeping it consistently maintained and updated.
Always check for software updates and follow the manufacturer’s recommendations to lengthen your car battery’s lifespan and improve its overall performance.
Conclusion
In conclusion, the components of an electric car battery cell are like members of a well-oiled machine, working together to power the revolution of transportation. From the anode and cathode to the separator and electrolyte, each element plays a crucial role in delivering efficient and sustainable energy. If cars were superheroes, then electric cars would be the ones with the coolest gadgets and secret weapons – their batteries.
So, the next time you see an electric car cruising down the street, remember that it’s not just a vehicle, it’s a masterpiece of science and engineering in action!”
FAQs
What are the main components of an electric car battery cell?
The main components of an electric car battery cell are the cathode, anode, electrolyte, and separator.
How do these components work together to power an electric car?
When an electric current is passed through the battery cell, the cathode and anode exchange ions through the electrolyte, creating an electrical charge that powers the vehicle.
What is the typical lifespan of an electric car battery cell?
The lifespan of an electric car battery cell varies depending on usage and maintenance, but they typically last between 8 to 10 years.
What are some common factors that can affect the performance of electric car battery cells?
Some common factors that can affect the performance of electric car battery cells include temperature, charging habits, and overall usage patterns.