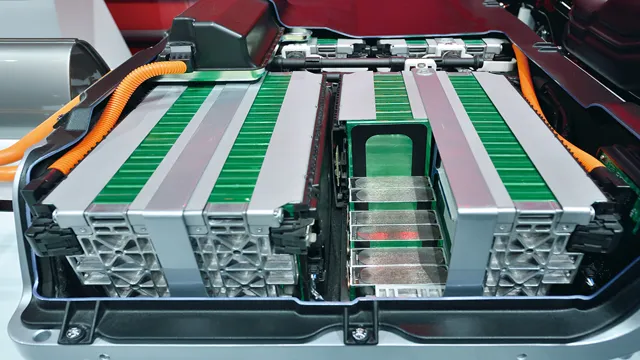
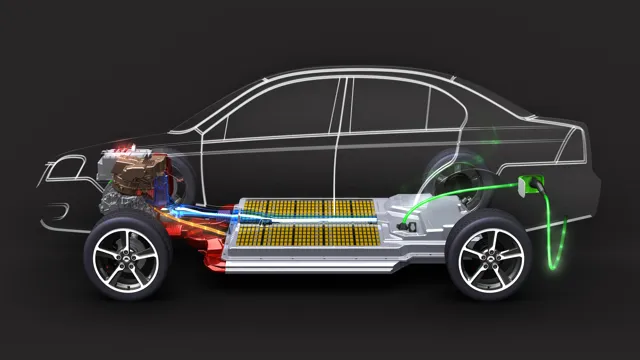
The Shocking Truth: The Vital Ingredients That Power Your Electric Car Battery
Electric cars are becoming more prevalent each year, and with them comes the advancement of their batteries. Electric car batteries are becoming more efficient and powerful, making them a top contender for the future of the automobile industry. However, have you ever wondered what makes up these batteries? What ingredients go into the creation of these powerful energy sources? In this blog, we will explore the various ingredients that make up the electric car battery and how they work together to power these eco-friendly modes of transportation.
Get ready to dive in and explore the world of electric car batteries!
Overview
Electric car battery ingredients are crucial components of any electric vehicle, and their efficiency can make or break the car’s performance. The most common ingredients used in electric car batteries include lithium, nickel, cobalt, and graphite. These elements are combined in various proportions and then used to create the battery cells.
While some ingredients are more expensive than others, manufacturers strive to find a balance between cost-effectiveness and performance. The battery’s charging capacity, range, lifespan, and safety are all factors that depend heavily on the ingredients used. As demand for electric vehicles grows, manufacturers are exploring alternative ingredients to reduce their reliance on rare and expensive materials.
Ultimately, the success of electric car batteries depends on a delicate balance of ingredients, technology, and innovation.
What are electric car batteries made of?
Electric car batteries are made up of several different materials that work together to provide power to the vehicle. The most important component of any electric car battery is the cathode, which is typically made from a combination of lithium, cobalt, and nickel. The anode, on the other hand, is often made from graphite, though some newer batteries use silicon instead.
The electrolyte, which is the substance that allows ions to move between the cathode and anode, is typically made from a liquid or gel-based solution. Finally, the casing of the battery must be made from a strong, durable material that can withstand the heat and pressure generated by the battery. While these materials may seem simple, they must be carefully chosen and combined in order to create a battery that can provide enough power to propel an electric car on the road.

Key ingredients in electric car batteries
Electric car batteries are made up of several key ingredients that work together to provide power. The main component of these batteries is the lithium-ion cell, which is made up of a cathode, an anode, and an electrolyte. The cathode is usually made of a combination of metals such as cobalt, nickel, and manganese, while the anode is typically made of graphite.
The electrolyte, which allows ions to move between the cathode and the anode, is usually made of a solution containing lithium salts. Other materials that are used in the production of electric car batteries include copper, aluminum, and various types of plastic. These components must be carefully balanced in order to create a battery that is both efficient and durable.
When it comes to electric cars, the battery is the most critical component, and the quality of the battery can greatly affect the performance and range of the vehicle. As demand for electric cars continues to grow, research into battery technology is becoming increasingly important, and new materials and manufacturing processes are being developed to create batteries that are even more efficient and long-lasting.
Lithium-ion Batteries
Electric car battery ingredients are a crucial factor in determining the performance of lithium-ion batteries. Lithium-ion batteries are rechargeable and widely used in electric vehicles because of their high energy density and long life. The three main ingredients of a lithium-ion battery are lithium, cobalt, and graphite.
Lithium is the lightest metal in the periodic table and is highly reactive, making it a good choice for battery electrodes. Cobalt, on the other hand, is used to stabilize the energy output of the battery, while graphite serves as the anode in the battery. Electric car manufacturers are constantly striving to improve the performance of their lithium-ion batteries by altering these ingredients.
For instance, nickel is used as a substitute for cobalt to lower the cost of the batteries and increase their energy density. Replacing graphite with silicon can also increase the battery’s energy density, although this may lead to shorter battery life. Overall, the choice of ingredients in electric car batteries is crucial in determining their strength, durability, lifespan, and cost-effectiveness.
Composition of lithium-ion batteries
Lithium-ion batteries are a commonly used type of rechargeable battery that has a high energy density and a low self-discharge rate, making them ideal for use in electronic devices such as laptops and smartphones. These batteries are typically made up of several components, including a positive electrode made of lithium cobalt oxide, a negative electrode made of graphite, and an electrolyte solution consisting of lithium salts in an organic solvent. The electrolyte solution acts as a medium for the transport of lithium ions between the positive and negative electrodes during charging and discharging cycles.
Additionally, separators are used to prevent internal shorts and to maintain a safe distance between the electrodes. Together, these components allow lithium-ion batteries to efficiently store and release energy to power our everyday devices.
Role of lithium in batteries
Lithium-ion batteries are the most commonly used rechargeable batteries, powering everything from smartphones to electric vehicles. The role of lithium in these batteries is critical to their performance. Lithium is a highly reactive element that can easily lose or gain electrons, making it an ideal material for storing energy.
The positive electrode of a lithium-ion battery is typically made of lithium cobalt oxide, while the negative electrode is made of graphite. During charging, lithium ions move from the positive electrode to the negative electrode, where they are stored in the graphite layer. When the battery is discharged, the lithium ions move back to the positive electrode, releasing their stored energy.
Lithium-ion batteries offer several advantages, including high energy density and low self-discharge rates. However, they also pose certain safety risks, as they are prone to overheating and catching fire if damaged or overcharged. Despite these drawbacks, lithium-ion batteries remain the most popular and widely used rechargeable battery technology today.
Other important battery components
Lithium-ion batteries are incredibly popular due to their high energy density and long lifetimes. However, there are other important components that make up a lithium-ion battery. One such component is the cathode, which is responsible for storing the positive charge of the battery.
The anode, on the other hand, stores the negative charge. Additionally, the electrolyte is a crucial part of the battery that facilitates the movement of ions between the cathode and the anode. Without all of these components working together, a lithium-ion battery would not function properly.
It’s important to consider all of the components when designing and manufacturing these batteries to ensure their efficiency and safety. So, the next time you use a lithium-ion battery-powered device, remember that there’s more to it than just the battery itself.
Lead-Acid Batteries
When it comes to electric car batteries, one of the most popular types used are lead-acid batteries. These batteries have been around since the 19th century and are still used in many applications today. The ingredients in a lead-acid battery include lead plates, sulfuric acid, and distilled water.
The lead plates are coated with lead dioxide and lead, which creates a chemical reaction when the battery is charged. The sulfuric acid serves as the electrolyte, which allows the flow of ions between the lead plates while the distilled water is used to dilute the acid. Despite their popularity, lead-acid batteries have their drawbacks.
They have a short lifespan and require regular maintenance, such as adding water and cleaning the terminals. Additionally, they are heavier and less efficient than newer battery technologies. That being said, they remain a reliable and cost-effective option for some applications.
Ingredients in lead-acid batteries
Lead-acid batteries are commonly used in various applications, including automobiles, boats, and backup power supplies. These batteries contain lead and sulfuric acid as their primary ingredients, along with other materials such as plastic, rubber, and lead alloys. The lead serves as the cathode, while the sulfuric acid acts as the electrolyte, enabling the flow of electrons between the cathode and the anode.
Lead-acid batteries are rechargeable, allowing them to be used repeatedly, and their low cost and easy availability make them a popular choice for many applications. However, they have several drawbacks, such as their heavy weight, limited capacity, and low efficiency, which can make them unsuitable for certain uses. Nonetheless, lead-acid batteries remain an important component of many industrial and consumer electronics, and their continued development and improvement will undoubtedly lead to even more widespread use in the future.
How lead-acid batteries work
Lead-acid batteries are a common form of rechargeable battery used in many applications, from cars to backup power systems. Inside these batteries are two lead plates submerged in sulfuric acid electrolyte. When a load is connected to the battery, a chemical reaction begins, with the lead plates reacting with the sulfuric acid to produce lead sulfate and hydrogen gas.
As the battery discharges, the lead sulfate builds up on the plates, reducing the battery’s capacity and ability to hold a charge. To recharge the battery, an external power source is connected, which forces the reverse chemical reaction, breaking down the lead sulfate and producing lead and sulfuric acid. Lead-acid batteries are reliable and inexpensive, but they do require periodic maintenance, such as adding distilled water to the electrolyte and removing any buildup on the lead plates.
It is important to properly dispose of old or damaged lead-acid batteries, as they contain hazardous materials that can be harmful to the environment.
Conclusion
So in conclusion, the secret to a successful electric car battery lies in its ingredients. We’re not talking about flour and sugar here, but rather a mix of lithium, cobalt, nickel and other rare elements that combine to create the power source of the vehicle. It’s like a chemistry experiment on wheels! And let’s be honest, who needs a gas-guzzling combustion engine when you have all these electrifying ingredients at your disposal? So next time you see an electric car zooming past, just remember that its power comes from a clever concoction of essential elements that’s changing the way we drive.
You might say it’s a electrifying revolution in the automotive industry, and we can’t wait to see where it takes us next!”
FAQs
What are the primary ingredients that make up an electric car battery?
The three main ingredients that make up an electric car battery are lithium, cobalt, and nickel.
How do these ingredients work together to create the battery’s performance?
Lithium acts as the battery’s main energy source, while cobalt and nickel help regulate the flow of energy and increase the battery’s overall durability.
Are there any environmental concerns associated with the use of these ingredients in electric car batteries?
Yes, there are concerns surrounding the mining and disposal of these materials, as well as potential ethical concerns surrounding the sourcing of cobalt in particular.
Are there any alternative materials that could be used in electric car batteries in the future?
Yes, researchers are exploring alternative materials such as solid-state batteries that use ceramics or polymers instead of liquid electrolytes, as well as using more abundant materials like sodium or potassium instead of lithium.