Revving Up Your Knowledge: Decoding the Electric Car Battery Reaction Diagram
If you’ve ever wondered what happens inside an electric car battery, you’re in the right place. The reaction that takes place inside the battery is what makes an electric vehicle a real contender for fossil fuel-powered vehicles. Electric car batteries are made up of several cells, each storing energy in the form of chemical compounds that convert to electrical energy when a circuit is created.
In this blog, I’ll take you on a journey inside the electric car battery, explaining the chemical reactions that take place and how they power the vehicle. Get ready to uncover the secret science behind the electric car battery and discover how it’s revolutionizing the automotive industry.
What is an electric car battery?
An electric car battery is the driving force behind the electric vehicle revolution. It is an essential component of an electric car that stores and releases electrical energy. This energy is used to power the electric motor that propels the car forward.
The battery is made up of multiple cells that are connected to form a single unit, and each cell contains electrodes made of different materials. During charging, chemical reactions take place in the battery, causing the electrodes to change chemically. The main keyword ‘electric car battery reaction diagram’ refers to the chemical process that takes place in the battery during charging and discharging, which involves the transfer of electrons between the electrodes.
This transfer of electrons creates current, which is used to power the car. When the battery is fully charged, it can provide enough energy to propel the car for hundreds of miles. However, as the battery discharges, its energy output decreases, and the car’s range reduces.
Therefore, battery technology is constantly evolving to improve the energy density, efficiency, and lifespan of electric car batteries.
Explaining the components
An electric car battery is the power source that propels the electric vehicle. It is the heart of the electric car, converting stored chemical energy into electrical energy needed to drive the motor. It is made up of several components, including the cathode, anode, electrolyte, and separators, all enclosed in a protective outer shell.
The cathode is the positive electrode and typically made up of lithium cobalt oxide, while the anode is the negative electrode and usually made of graphite. The electrolyte, such as lithium-ion, acts as a medium for electrical conductivity between the two electrodes, and the separators keep the cathode and anode separate. Unlike gasoline-powered engines, electrical batteries require regular recharging, and the range of the vehicle depends on the battery’s capacity.
Most modern electric cars use lithium-ion batteries, which are lighter, have higher energy density, and are more efficient than other battery options. With advancements in technology and the push towards sustainable energy solutions, electric car batteries are becoming increasingly popular as the world moves towards a greener future.
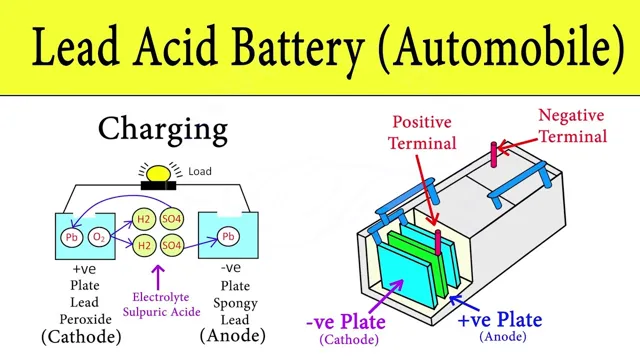
How it operates
An electric car battery is the power source for electric vehicles. It is a rechargeable lithium-ion battery that is used to store electrical energy to power the vehicle. The batteries are typically made up of multiple cells that are connected together to form a pack.
These cells contain a lithium-ion electrolyte that allows the battery to charge and discharge as needed. The electric motor in the car draws power from the battery pack to drive the wheels, and the battery is recharged through regenerative braking or by plugging the vehicle into an electrical outlet. Electric car batteries are designed to be long-lasting and are expected to continue operating for many years before needing to be replaced.
With advancements in technology, electric car batteries are becoming more efficient and able to provide longer ranges between charges, making electric vehicles a truly viable option for environmentally conscious drivers.
The chemical reaction within the battery
When you plug an electric car into a charging station, the chemical reaction within the battery is what makes it all possible. The process begins with lithium ions moving from the cathode to the anode, creating an electric current. This reaction is reversible, which is why you can recharge an electric car battery.
The anode is made of carbon and graphite, while the cathode is made of metal oxides. As the electrons pass through the battery, they create a flow of electricity that powers the electric car motor. It’s important to note that the battery chemistry is different for each type of electric car, which is why some models have longer ranges than others.
While there’s no single “electric car battery reaction diagram” that applies to all vehicles, the basic concept remains the same throughout the industry. In short, without this chemical reaction, electric cars would simply be unable to function.
Breaking down the process
When we use a battery-powered device, we often don’t think about what’s happening inside the battery to make it work. But breaking it down, there’s a complex chemical reaction taking place. The reaction involves the battery’s two electrodes: the cathode and the anode.
The cathode is typically made of a metal oxide, while the anode is usually made of a carbon-based material. When the battery is put into use, electrons begin to flow from the anode to the cathode through an external circuit. This sets off a chemical reaction that causes the metal oxide in the cathode to release its oxygen atoms.
The oxygen atoms then react with the carbon in the anode, producing carbon dioxide and releasing electrons in the process. These released electrons then travel back to the cathode, completing the circuit and powering our devices. The keyword used in this paragraph is “chemical reaction.
“
Visual representation of the reaction
When we use a battery, there is a lot going on behind the scenes. The chemical reaction happening inside the battery allows it to produce the energy that we rely on. The reaction is essentially a transfer of electrons from one substance to another.
In most batteries, one substance is a metal and the other is a chemical compound. When the battery is turned on, the metal releases electrons and they flow through a wire to the other substance. This flow of electrons creates a flow of current and provides the energy that we use.
It’s like a game of hot potato, where the electrons are passed from one substance to another until they reach their destination. This chemical reaction is what powers our devices and allows us to stay connected on the go. So next time you use a battery, remember the tiny chemical reaction that is making it all possible.
Impacts on battery performance
Battery performance can be affected by various factors, and one of the most significant is the chemical reaction within the battery. Battery chemistry plays a crucial role in determining the performance of the battery and how it responds to different conditions. The chemical reaction that occurs inside the battery involves the transfer of electrons from the anode to the cathode and vice versa.
This reaction generates the electrical energy required to power our electrical devices. However, over time, the chemical reaction can become less efficient, resulting in reduced battery performance. For instance, the chemical reaction rate can be negatively affected by high temperatures or low temperatures.
As the battery chemistry changes, the battery may lose its ability to hold charge for as long as it used to. This is why it’s important to keep batteries within their optimal operating temperature range and to avoid overcharging or deep discharging the battery. By paying attention to the chemical reaction within the battery, we can help prolong its life and maintain its performance.
Maintenance tips for your electric car battery
When it comes to maintaining your electric car battery, it’s important to understand how it works. Every battery contains an anode (negative electrode) and a cathode (positive electrode), separated by an electrolyte solution. The chemical reaction that takes place between these components allows the battery to produce an electric current.
However, certain factors such as temperature, charging habits, and overall usage can impact the battery’s performance over time. One of the best ways to keep your battery in good condition is to avoid fully charging or fully discharging it. Doing so can cause damage to the battery’s cells, reducing its overall durability and lifespan.
Additionally, keeping the battery at a moderate temperature and avoiding extreme temperatures can help ensure optimal performance. By following these maintenance tips and understanding the electric car battery reaction diagram, you can help extend the life of your battery and keep your electric vehicle running smoothly.
Preventing reduction in battery life
Electric car batteries have come a long way in recent years, but they still require proper maintenance in order to maximize their lifespan. One of the most important tips for preventing a reduction in battery life is to avoid overcharging. This means that you should not leave your car plugged in once the battery is fully charged.
Another important tip is to avoid letting your battery fully discharge. Allowing your battery to completely run out of power can have a negative impact on its overall health. Additionally, it’s important to keep your battery at a moderate temperature.
Extreme heat or cold can cause damage to the battery and reduce its lifespan. By following these maintenance tips, you can ensure that your electric car battery lasts as long as possible, saving you money in the long run.
Carrying out effective maintenance
Electric car battery maintenance is essential to keep your vehicle running smoothly. Here are some tips to help you maintain your electric car battery and increase its lifespan. First and foremost, ensure that you charge the battery fully after each use.
This will help keep your car running longer and make sure you have enough power for your next journey. Additionally, avoid exposing the battery to extreme temperatures, as high heat or cold can cause damage. When the weather is particularly hot or cold, it’s best to park your car in a shaded or covered area.
Lastly, have your battery checked regularly by a professional to catch any potential issues early on. By following these simple tips and taking proactive measures to maintain your electric car battery, you can ensure that your car performs optimally for years to come.
Conclusion
In conclusion, the electric car battery reaction diagram is like a puzzle that powers a vehicle. The chemical reactions that take place between the electrodes, electrolyte, and electrons come together to create an electric charge that can drive the car. This is a remarkable feat of engineering and science that continues to evolve and improve.
So the next time you see an electric car on the road, appreciate the intricate dance of chemistry that’s taking place beneath the hood.”
FAQs
What is an electric car battery reaction diagram?
An electric car battery reaction diagram represents the chemical reactions that occur inside an electric car battery to produce electrical energy.
Why is an electric car battery reaction diagram important?
An electric car battery reaction diagram helps us understand how an electric car battery works and how it produces electrical energy, which is essential for diagnosing and solving any issues with the battery.
What are the common types of chemical reactions in an electric car battery?
Electric car batteries mainly involve two types of chemical reactions: oxidation and reduction. In the oxidation reaction, the anode releases electrons, while in the reduction reaction, the cathode accepts these electrons.
How does an electric car battery reaction diagram differ from a traditional car battery diagram?
The chemical reactions in an electric car battery are different from those in a traditional car battery. Electric car batteries typically use lithium-ion cells, which involve complex multi-step reactions, while traditional car batteries use lead-acid cells, which involve simpler reactions. Additionally, electric car batteries have different requirements for charging and maintenance compared to traditional batteries.





